Pyruvate Dehydrogenase Complex is RAD
-
And by RAD I mean 'regulated as drugs'.
This enzyme complex belongs to the α-ketoacid or 2-oxoacid dehydrogenase complex family:
- Pyruvate Dehydrogenase Complex [PDHc]
- Pyruvate → Acetyl-CoA (+ CO₂)
⠀
- Pyruvate → Acetyl-CoA (+ CO₂)
- Ketoglutarate (or Oxoglutarate) Dehydrogenase Complex [KGDHc]
- Ketoglutarate → Succinyl-CoA (+ CO₂)
⠀
- Ketoglutarate → Succinyl-CoA (+ CO₂)
- Branched-chain Ketoacid Dehydrogenase Complex [BCKDHc]
- Leucine →→ Isovaleryl-CoA (+ CO₂)
- Isoleucine →→ Methylbutyryl-CoA (+ CO₂)
- Valine →→ Isobutyryl-CoA (+ CO₂)
- *'Methionine' or 'Threonine' →→ Propionyl-CoA (+ CO₂)
- *May also be metabolized by PDHc.
⠀
- *May also be metabolized by PDHc.
- Ketoadipate (or Oxoadipate) Dehydrogenase Complex [KADHc]
- Lysine or Tryptophan →→ Glutaryl-CoA (+ CO₂)
Because of the greater complexity of PDHc, understanding its basics should make the others seem relatively straightforward.
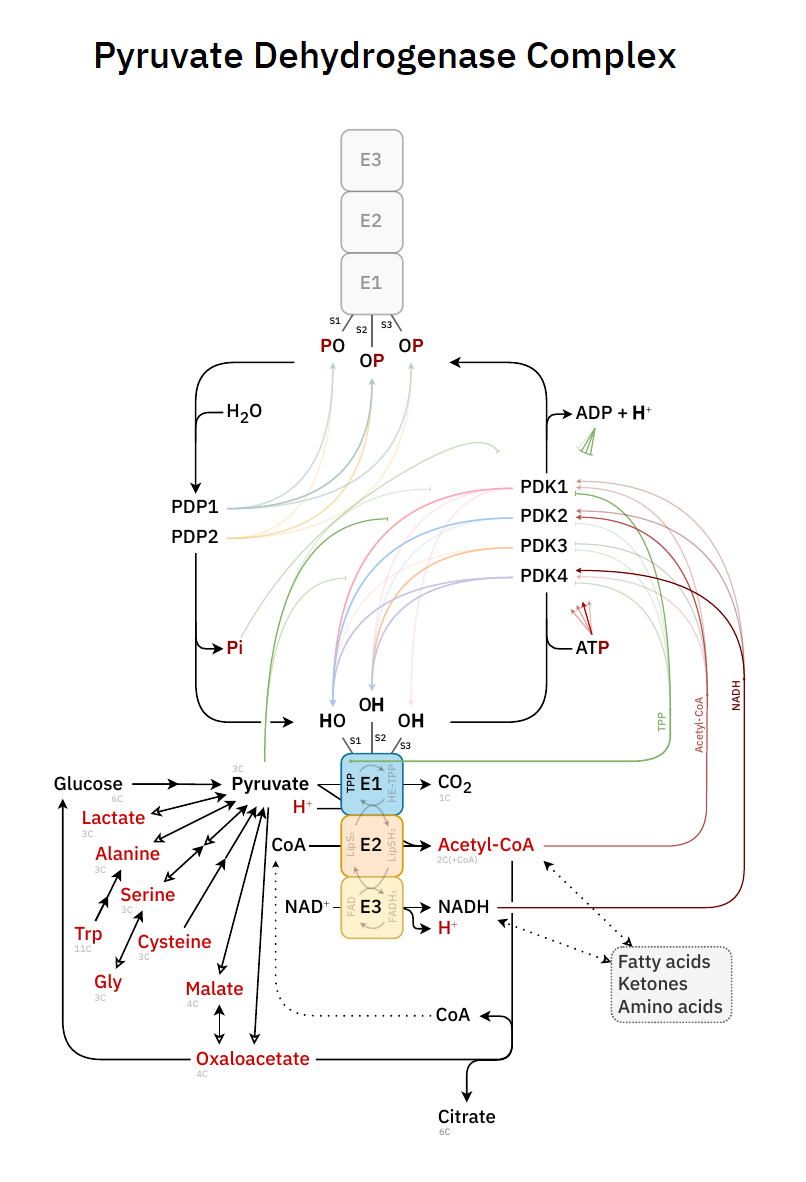
Pyruvate Dehydrogenase Complex has 3 main components:
- E1: pyruvate decarboxylase * (
PDHTTP) - E2: dihydrolipoamide acetyltransferase (DLAT)
- E3: dihydrolipoamide dehydrogenase (DLDH)
*On naming E1:
"The recommended trivial name for [E1] is pyruvate dehydrogenase [PDH], but to avoid confusion with pyruvate dehydrogenase complex [PDHc] we have used the name pyruvate decarboxylase for [E1]. Because of this confusion, P.J.R. has mistakenly referred to the complex [PDHc] as [PDH] in a number of earlier papers."
(10.1042/bj1730659)
If Phil confused them in publications, we might do it too. However, a separate yeast pyruvate decarboxylase exists, and so does a bacterial pyruvate oxidase, making it tricky to pick the least confusing term for this enzyme responsible for oxidative decarboxylation. Alternatively, we may stick to E1 or go with TTP, for 'that thing of PDHc'.
The 3 components listed are the ones that are often featured in figures:
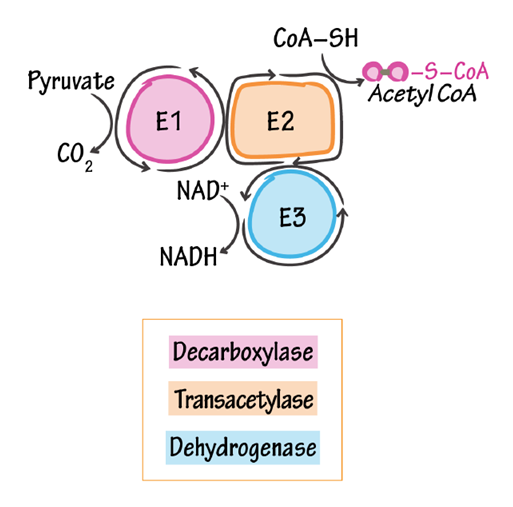
PDHc contains an additional structural component:
- E3BP: dihydrolipoamide dehydrogenase–binding protein
And also a few instances of the regulatory enzymes:
- PDK: Pyruvate dehydrogenase kinase
- PDP: Pyruvate dehydrogenase phosphatase
A scheme of a basic unit of PDHc with cores in evidence:
⠀(978-0-12-540070-1)
You may not know this, but Kvothe got into animes because of a South Korean girlfriend that he once had. She used send him pictures with a peculiar 'finger heart' gesture..

..that reminded him how the two dimers of E1 assemble:
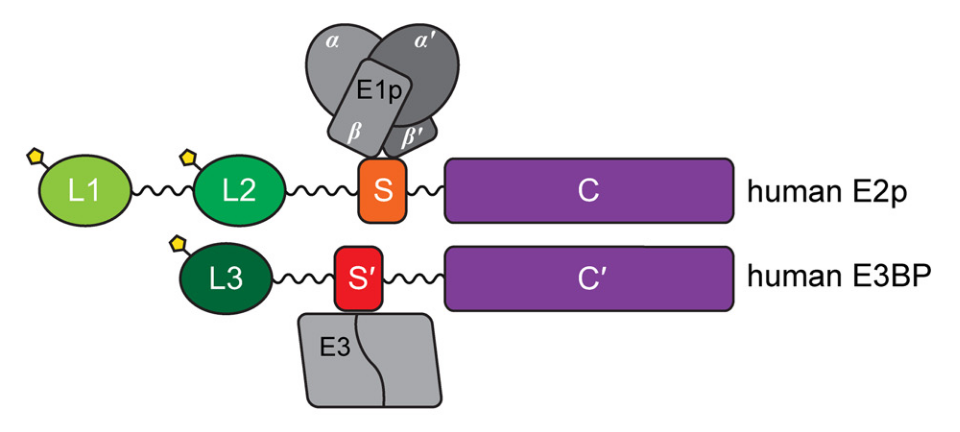
⠀(10.1074/jbc.R114.563148)
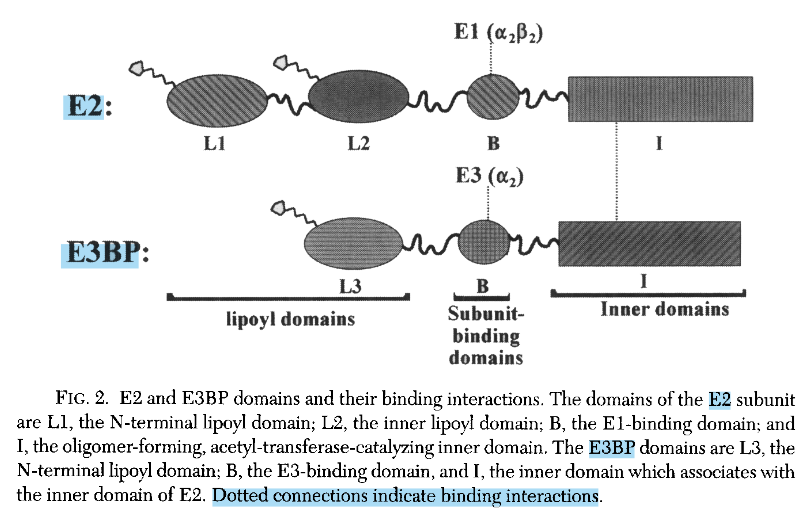
Green Orange/Red Purple Lipoyl Domains Subunit-Binding Domains Catalytic Inner Domains E2 and E3BP are similar core proteins, but with some noteworthy differences:
E2 E3BP 2 lipoyl domains 1 lipoyl domain Binds E1 Binds E3 Catalytic/Enzymatic inner domain ('acetyltransferase') Non-enzymatic inner domain (mutated and dummy) No surprise that a 'E3-binding protein' binds E3.
E3 processes the same reduced lipoyl domains in last-stage reactions and is common to all ketoacid dehydrogenase complexes, but E1 and E2 deal with unique metabolites, making them more specific. Due to their specificity, sometimes you'll come across the following identifiers:
- E1p, E2p, E3: pyruvate..
E1o, E2o, E3: oxoglutarate..E1b, E2b, E3: branched-chain ketoacid..
'E1p' in the figure above would be an example.
For a slightly more realistic scheme, that includes the regulatory enzymes:
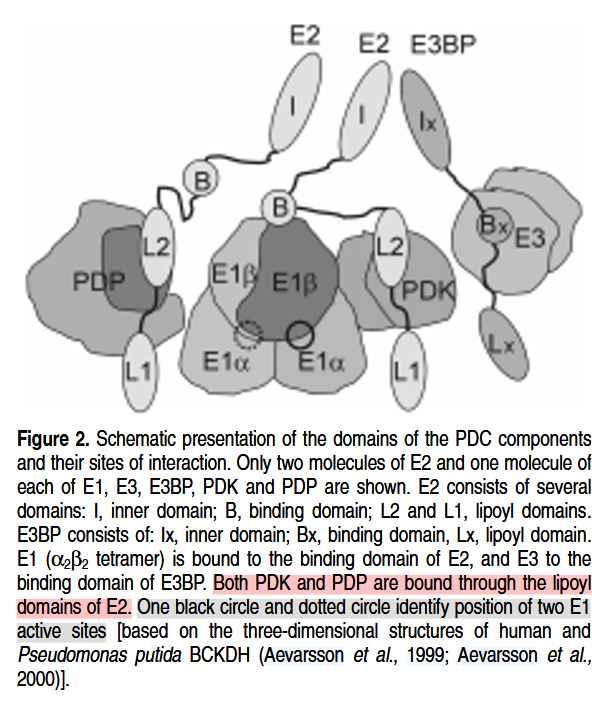
⠀(978-0-12-540070-1)
But the figure represents only a fraction of the complex. Whole PDHc is massive, made of multiple recurring parts:
Component Subunits/PDHc Subunit composition E1 ~20 α₂β₂ E2 1 48α E3BP 12 - E3 ~6 2α PDK 1–3 αβ PDP 2–3 αβ The values can vary, but this gives a rough idea of its size and complexity.
The core subunits (E2s exclusively or E2s and E3BP) combine through their inner domains in trimers, matching how the authors chose to display them on the previous image (⇈ I + I + Ix). Many of these trimers (20 of them) join to form a pentagonal dodecahedron (12-face geometry) that represents the central core of the complex:
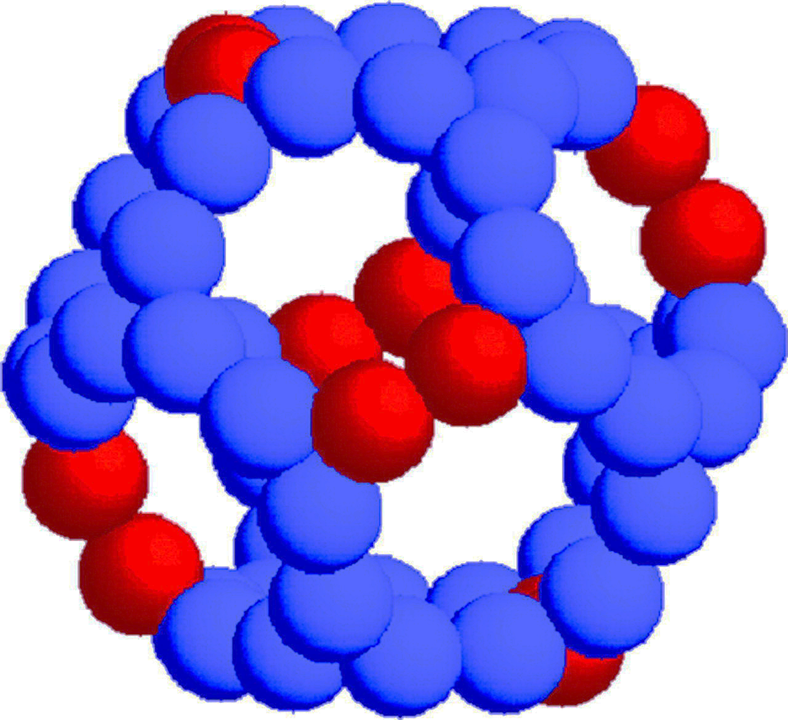
⠀(10.1074/jbc.M308172200)
- Blue: E2 inner domain
- Red: E3BP inner domain
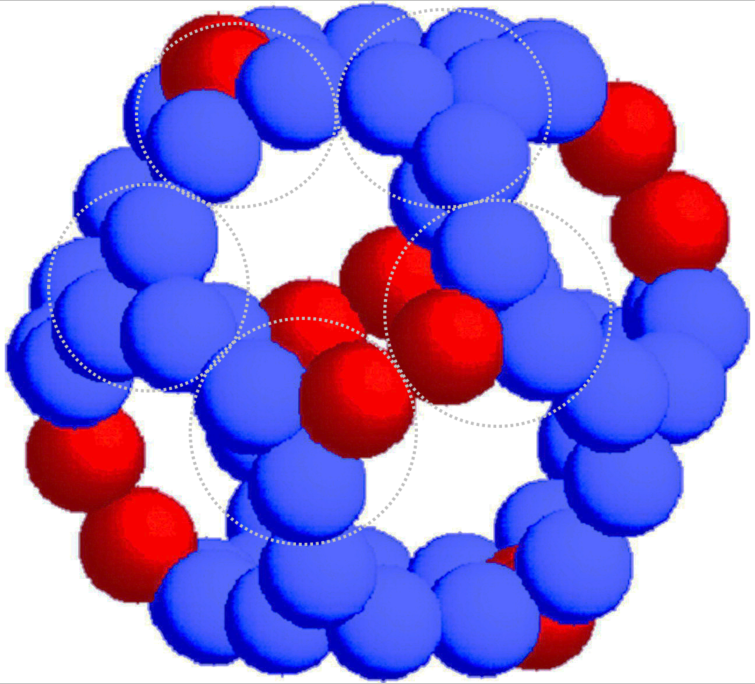
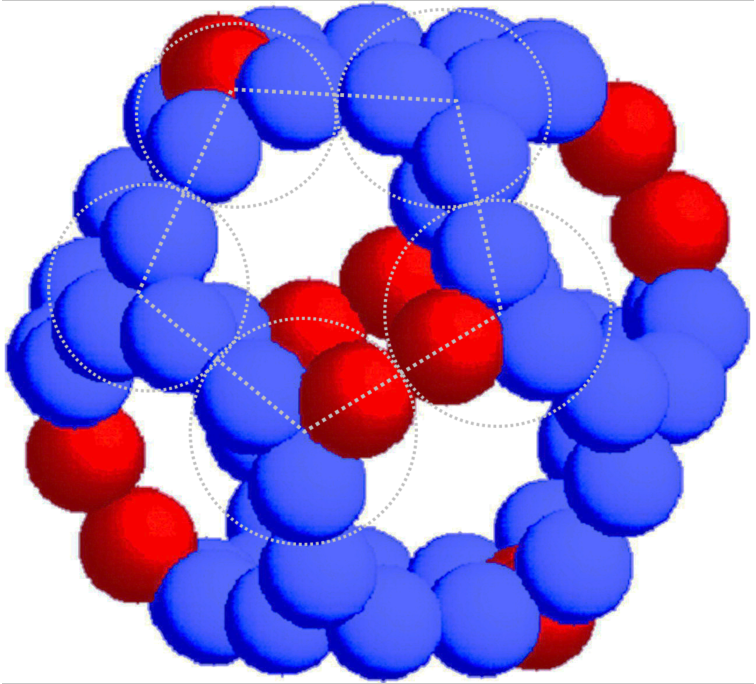
Therefore, the central core is made of E2 subunits with occasional substitutions with E3BP.
⠀(10.1016/j.str.2019.04.009)
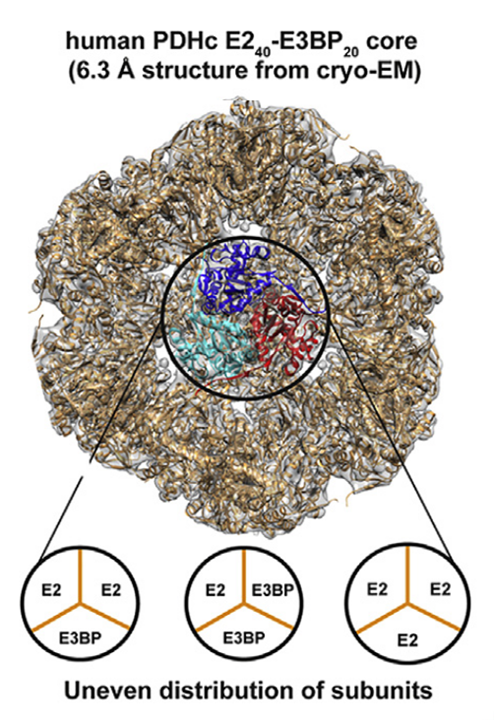
The E2:E3BP ratio per
trimerwhole PDHc is a matter of debate, but..- 40–48 E2
- 20–12 E3BP
..for a total of 60 core subunits (as in the composition table) are possible scenarios.
Knowing that:
- E1 binds to E2
- E3 binds to E3BP
Based on average values, we may have the subunits in the following proportions:
- (20E1/48E2)/PDHc
- (4E3/12E3BP)/PDHc
The ratio of E1 or E3 per binding subunit is less than 1 (<1:2) in both cases, showing how unoccupied E2 and E3BP tend to be, and also how both have the potential to bind more E1 and E3 if needed for PDHc to increase activity.
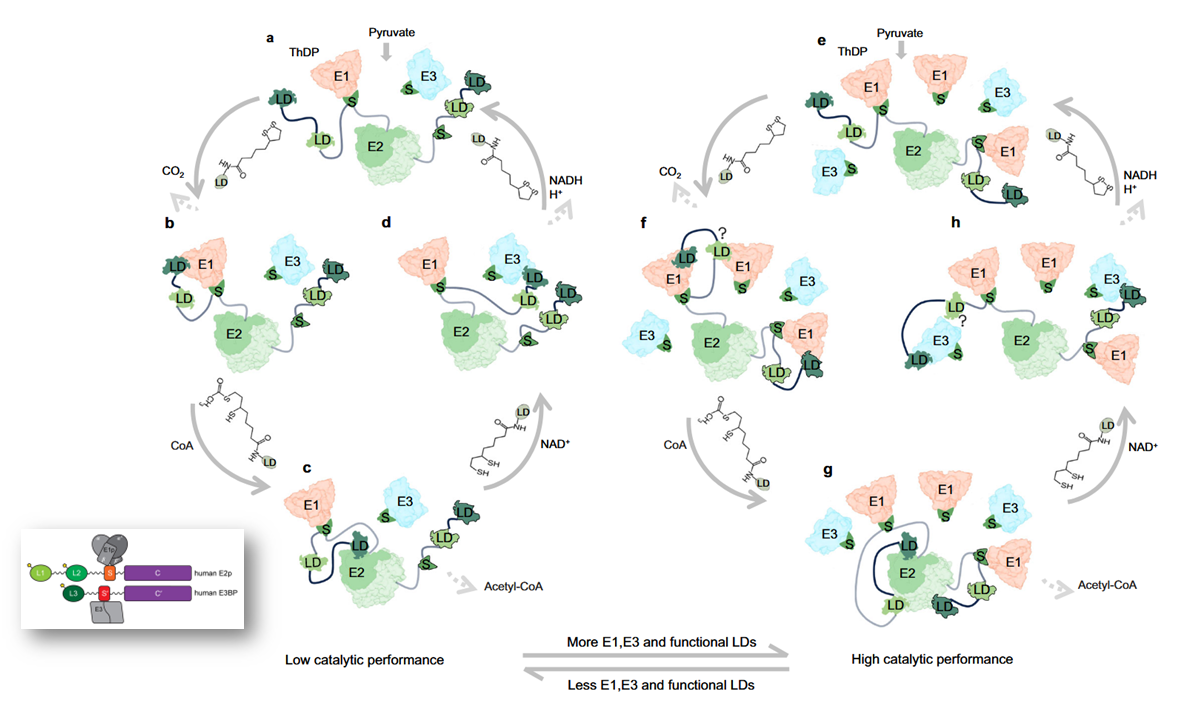
⠀(10.1038/s41467-025-56171-8)
Lipoyl domains L1 and L3 are not as critical for PDHc function as L2; their elimination in experiments doesn't compromise PDHc to the same extent as L2. Nevertheless, the figure suggests that lipoyl domains occupancy might as well increase in a supportive way: "more E1, E3, and functional LDs" if needed.
The quantity of E1 per PDHc is also much higher than E3, a relationship that remains present in optimal conditions, likely compensatory for the limiting reaction of PDHc that occurs in E1.
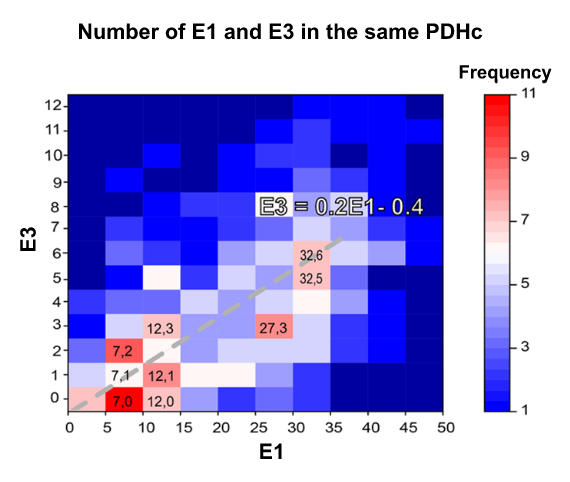
⠀(10.1038/s41467-025-56171-8)
Even though the number of E2 per PDHc is higher than that of E1, E2 has a structural role.
A view of the complex without linkers:
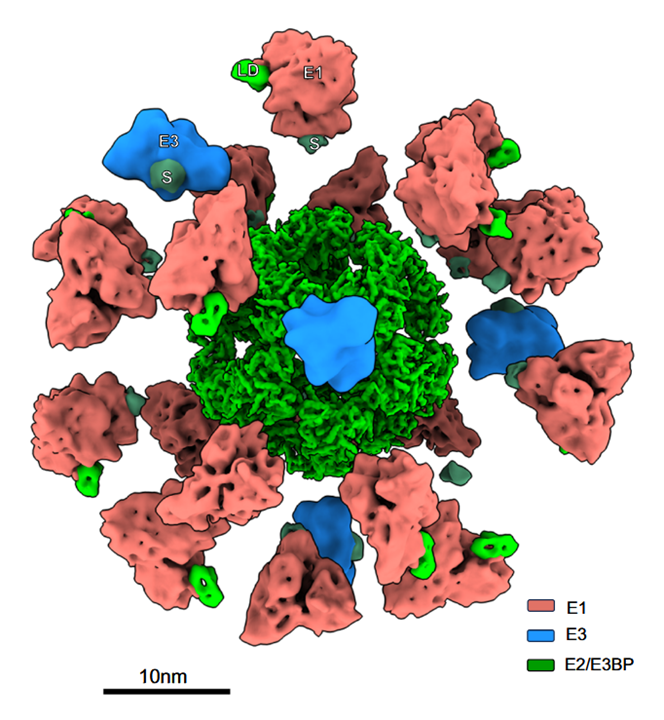
⠀(10.1038/s41467-025-56171-8)
And here highlighting a trimer with the linkers stemming from their inner domains:
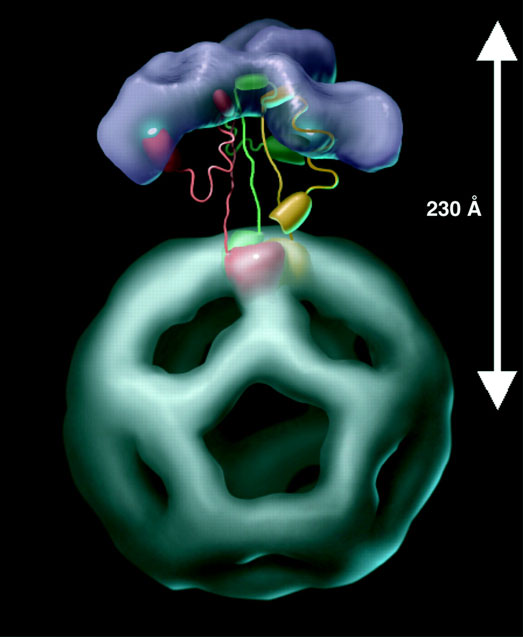
⠀(10.1093/emboj/cdf574)
'Red, green and yellow' colors at the base highlight each core subunit of the trimer.
The large structures over the central core would be E1 (and E3) subunits anchored through the binding domains, that in turn function as pivots for the swinging arms:
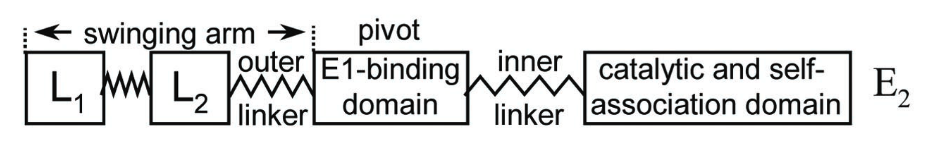
⠀(10.1073/pnas.011597698)
Relative to the pivot point, the left part is longer than the right one, allowing the lipoyl domain to reach not only the E2 catalytic domains of the anchoring trimer, but also those of trimers nearby. For the same reason, the lipoyl domain can react with neighboring E1s, not just the one sharing the same core. How this cooperativity would work:
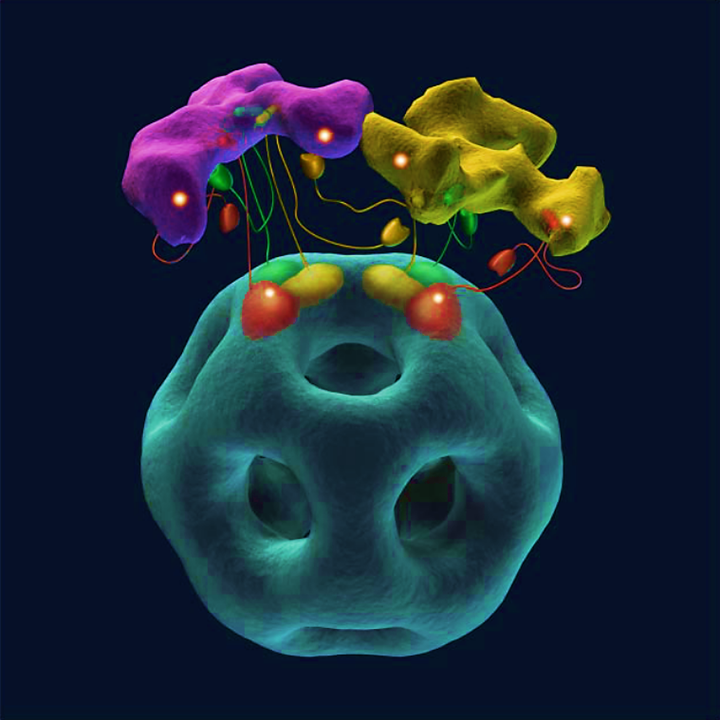
⠀(10.1074/jbc.M504363200)
The shinning spots are the reaction centers.
The swinging arms move the most, but the rest of the complex is not static, although the movement of other parts is limited by the inner linkers that are relatively rigid. Getting closer to center would also imply packing bulky structures, which could lead to their conflict. Nevertheless, it's all movable, including the central core, that can expand and contract.
With this background information, the following animation should become more meaningful:
- Pyruvate Dehydrogenase Complex [PDHc]
-
In the PDHc subunit composition table, you might have noticed that only a few regulatory enzymes occur per complex (≤3 PDK or PDP per PDHc). These enzymes are typically bound to lipoyl domains, but can hop between lipoyl domains from different cores, so clusters of E1s should maximize the efficiency of PDK or PDP.
PDK adds and PDP removes a phosphate group from specific serine residues (termed phosphorylation sites) of the E1 subunit, which leads to inactivation and reactivation of PDC. This reversible phosphorylation is not the only regulatory modification, but it's a major one. If you want to traumatize your way into remembering it: PDKilnase 'kills' the activity of PDHc, leaving for PDP to 'promote' it.
- PDHc: the complex has various E1 enzymes
- E1: a heterotetramer (α₂β₂)
- E1α: each of the two α-subunits of E1 has 3 phosphorylation sites to regulate PDHc as drugs
- E1: a heterotetramer (α₂β₂)
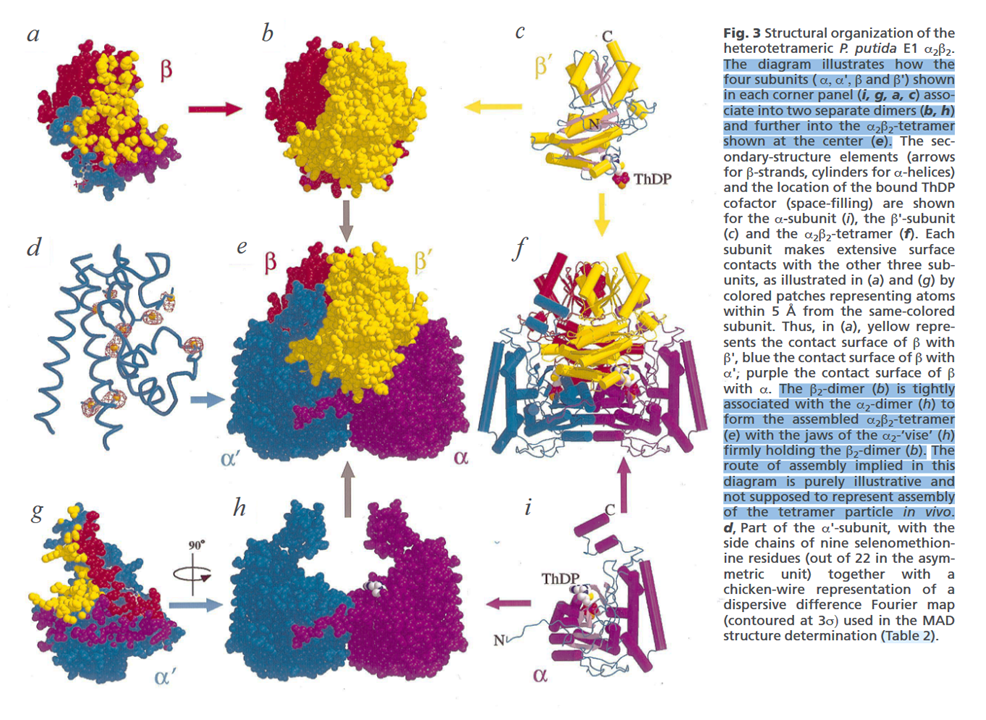
⠀(10.1038/11563)
⠀(10.1038/emm.2001.32)
Totaling 6 phosphorylation sites (three distinct pairs) per E1.
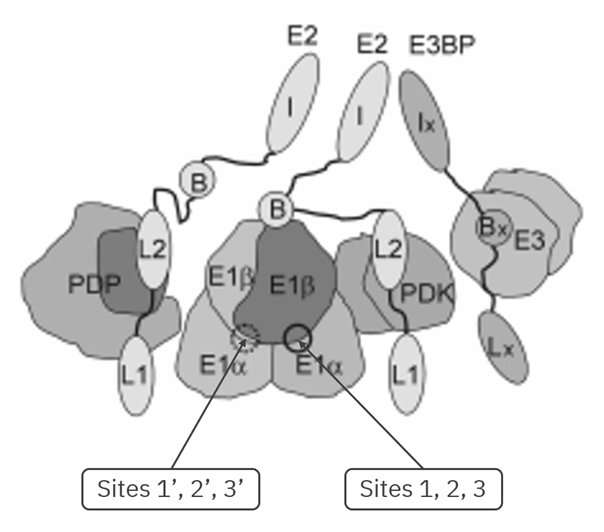
PDKs (and PDPs) are large and tend to associate with lipoyl domains to enhance their (de)phosphorylating function. The target sites are in E1, that has a flattened pear shape, and occurs tilted from the base towards the nucleus. Differences in orientation may give one of the targetable sides greater exposure to the anchored regulatory enzymes.
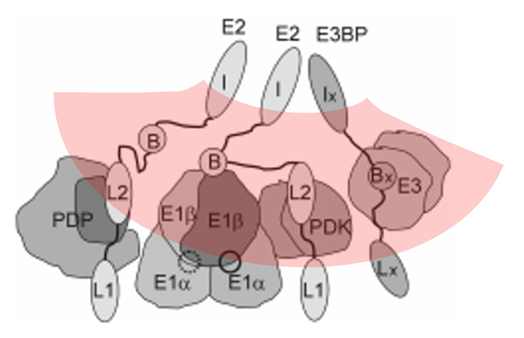
⠀(10.1038/emm.2001.32)
The outer lipoyl domain (L1) can cover a longer range, but PDKs and PDPs have a preference for associating with L2. Despite the increased efficiency, this anchoring might cause PDK to favor targeting the region with greater exposure, while compromising access to the other (example from the cartoon:
 solid circle
solid circle  dotted circle), halving the phosphorylation capacity. Regardless of what's behind the minimal phosphorylation redundancy, for a total of 6 phosphorylation sites per E1, no more than 3 sites are regarded targetable.
dotted circle), halving the phosphorylation capacity. Regardless of what's behind the minimal phosphorylation redundancy, for a total of 6 phosphorylation sites per E1, no more than 3 sites are regarded targetable.Even though the phosphorylation of these specific serine residues makes up the primary regulatory modification, PDHc can undergo phosphorylation on alternative residues as well. PDHc may also be subjected to modifications beyond acetylation and phosphorylation, such as glycosylation, glutathionylation, nitrosylation, succinylation, and possibly others. In addition, long-term adaptations can involve changes in the occurrence of select enzymes.
The main regulatory enzymes have specific isoforms:
-
PDKs: Pyruvate dehydrogenase kinases
- PDK1, PDK2, PDK3, PDK4
-
PDPs: Pyruvate dehydrogenase phosphatases
- PDP1*, PDP2
- *Catalytic and regulatory variants
- PDP1*, PDP2
They differ in tissue expression, activity, sensitivity to effectors, and preferential target sites. This adds a layer of control for the body to express a purposeful combination depending on needs.
Each graph below represents an isoform of PDK (1–4), with their respective P/E1 ratios (phosphates incorporated per E1 subunit):
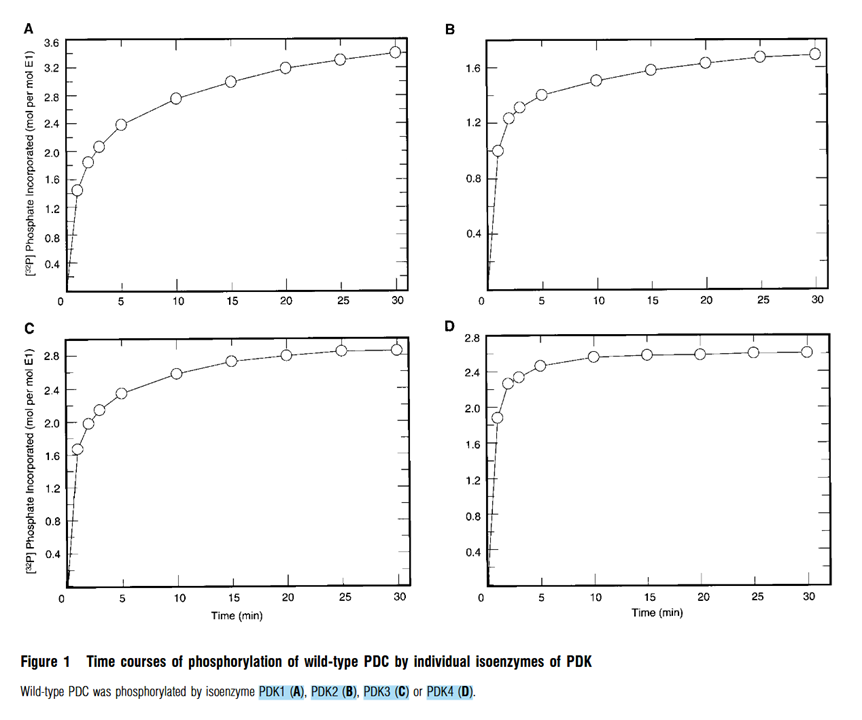
⠀(10.1042/bj3580069)
In leveling off and ignoring the decimals, we have:
Isoform Phosphorylation degree PDK2 1P/E1 PDK3, PDK4 2P/E1 PDK1 3P/E1 This is how much phosphorylation we can expect from each isoform, but yet without detailing their preferential target sites.
Nevertheless, the initial phosphorylations are responsible for most of the inactivating effect--with site 1 being the most and site 3 the least prone to targeting--and a single occupied site can be enough for a near complete inactivation of the complex.
Site 1 occurs at a critical stabilizing location to the order of E1, making it not only a favored site, but also the most disruptive to the overall PDHc catalytic activity. Independent of the targeted site, the higher the degree of phosphorylation, the more PDHc resists reactivation by PDPs. Sustained signals to divert pyruvate from oxidation favor hyperphosphorylation.
An example of liver and heart responding differently to refeeding after fasting:
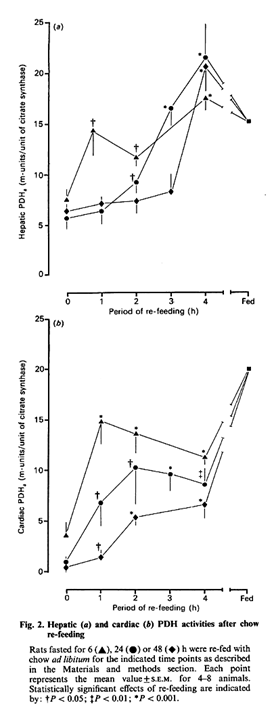
⠀(10.1042/bj2580529)
The reactivation of heart PDHc was slower with longer fasting, and the additional phosphorylation from the greater occurrence of PDK1 characteristic of the organ possibly contributed to this delay.
High-fat diets, leading to an oversupply of fatty acids, are also inhibitory. However, low-carbohydrate diets are lesser of a threat than fasting, which might be a factor in the faster response in human muscles to carbohydrate-rich refeeding:

⠀(10.1152/ajpregu.90604.2008)
But even in healthy and carbohydrate-replete conditions, PDHc isn't fully active. Some level of inhibition persists, leaving a margin for further activation (or disinhibition) if suddenly needed.
PDHc is a decisive point in glucose metabolism; not because it always commits glucose to complete oxidation, but because PDHc decarboxylates pyruvate (3C) to acetyl (2C) in an irreversible reaction. If this acetyl group entered the TCA cycle, advancing the cycle to arrive on oxaloacetate (a glucose synthesis precursor) would eliminate the 2 gained carbons (−2C), making it futile to rely on acetyls to resynthesize glucose.
When the equivalent of an acetyl group is completely eliminated after a full 'turn' of the TCA cycle, the cycle returns to its original state, with oxaloacetate ready to condense with a new acetyl group.
Oxaloacetate (4C) + Acetyl (2C) → Citrate (6C)
In a situation where acetyl groups occur in excess, pyruvate can relieve this excess by converting into oxaloacetate (via PC) instead of acetyl (via PDHc).
Pyruvate (3C) ↛ Acetyl (2C)
↳ Oxaloacetate (4C) –↳ Citrate (6C)
The pyruvate reroute helps to avoid further accumulation of acetyl groups, but now the body has to deal with extra citrate. After all, if oxaloacetate is recycled in the TCA cycle, its continuous regeneration should have supported the catabolism of incoming acetyl groups.
Citrate is normally metabolized in the TCA cycle, but it can also be diverted for lipid synthesis or acetylation. Acetyl groups are produced bound to CoA, a large molecule that can't cross compartments. Whether pyruvate enters the TCA cycle as acetyl-CoA or oxaloacetate, once converted into citrate, the acetyl groups surplus becomes exportable. After exported, they can be recovered in the cytosol in an energy-consuming reaction. Despite the hassle, oxaloacetate is regenerated together with acetyl-CoA and may return to keep the cycle running:

⠀(10.1038/s41586-022-04475-w)
This malate-citrate shuttle can be substituted by the known malate-aspartate shuttle to not condition the import of malate to the export of citrate (SLC25A1 works as an exchanger), allowing the body to recover oxaloacetate either way despite the diversion.
Whether it's derived from glucose or fatty acids, too much citrate (or derivatives) leaving the mitochondria signals energy surplus and is inhibitory to the competing substrate:
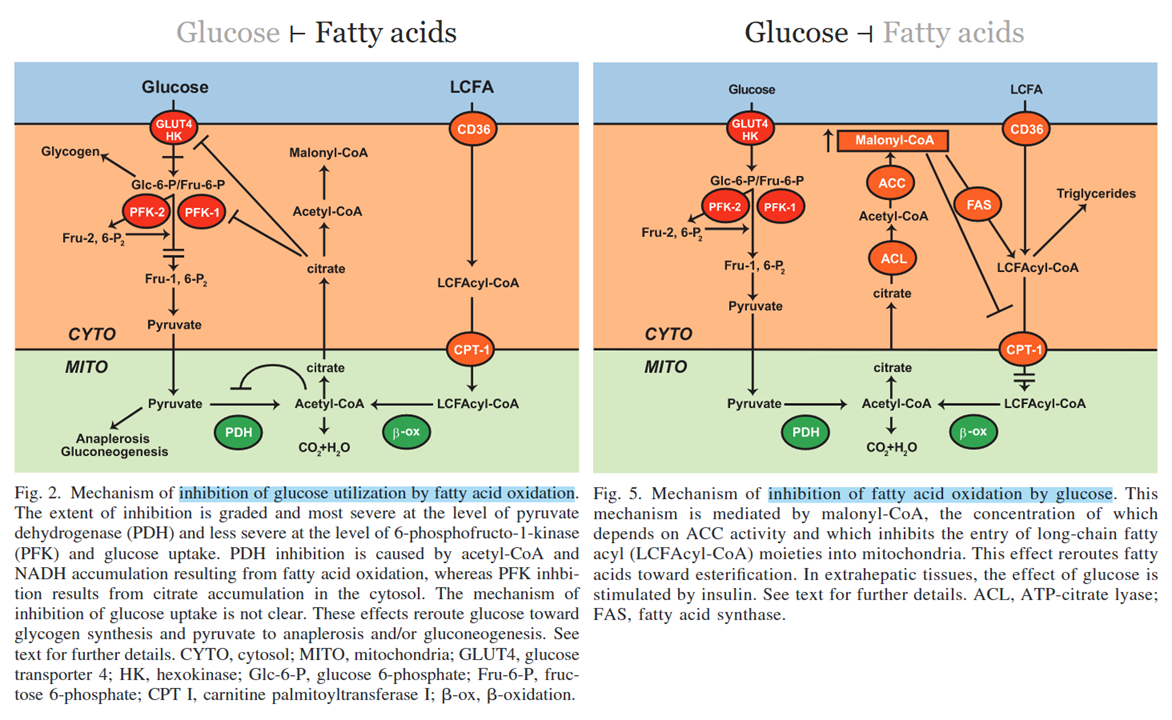
⠀(10.1152/ajpendo.00093.2009)
When products in common with other pathways accumulate to the point of inhibiting PDHc, it raises the question of whether the accumulation is a result of overproduction from competing substrates or impaired consumption as a consequence of some blockage downstream.
Succinate dehydrogenase [succinate —(SDH)→ fumarate] is in a privileged position for metabolic control in mitochondria because the enzyme is shared by the TCA cycle and Electron Transfer System (ETS), where SDH is alternatively referred to as Complex II.
A mismatch in the rate of TCA cycle to ETS can lead to succinate formation in excess of its oxidation. If succinate accumulates, it can reinforce PDHc inhibition indirectly by preventing the continuous degradation of Hypoxia-inducible Factor 1α (HIF-1α), which hints at the default behavior of the impaired cell, before the supply is cut.
"Succinate is an inhibitor of prolyl hydroxylase (PHD), another alpha-ketoglutarate(αKG)-dependent dioxygenase [43], which is responsible for hydroxylation of hypoxia-inducible factor 1-alpha (HIF1α) causing its degradation. Then, succinate accumulation results in a pseudo-hypoxic response that is caused by HIF1α stabilization and activation of genes containing HIF response elements (HREs), many of them involved in glycolysis such as glucose transporter 1 (GLUT1), HK2, LDHA, pyruvate dehydrogenase kinase isozyme 1 (PDK1) [8,42]." (10.1016/j.semcdb.2019.04.013)
↓ETS, ↑Itaconate, etc. → ↓SDH → ↑Succinate → ↑HIF-1α → ↑PDK1 → ↓PDHc
When PDHc is inhibited in a condition such as cancer, glucose and glutamine can still converge in succinate:
- Glucose →→ pyruvate → oxaloacetate → malate → fumarate → succinate
- AAs → glutamine → glutamate → ketoglutarate → succinyl-CoA → succinate
Succinate accumulation is one of the many examples of potential downstream problems that can affect PDHc. However, considering that pyruvate..
- is a convenient electrons acceptor for cytosolic NAD⁺ regeneration,
- may function as an antioxidant in itself, and
- can be a source of reactive oxygen species (ROS),
..forced pyruvate oxidation via PDHc can be exploited therapeutically in spite of downstream problems, and it can have a destabilizing effect in cancer cells.
Ketoacid dehydrogenase complexes are not the main producers of ROS, but they are overlooked sources.
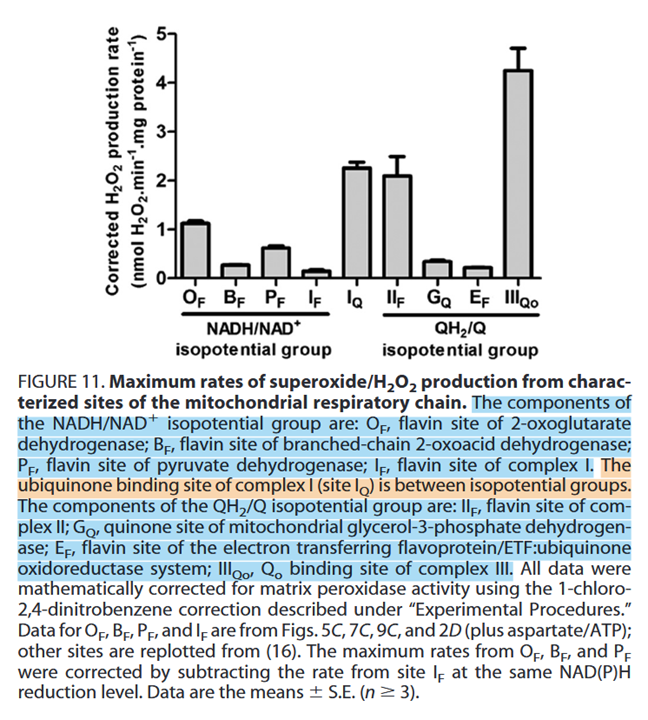
⠀(10.1074/jbc.M113.545301)
Flavins participate in one-electron reactions and can create reactive species. It's problematic 'F' all around.
Despite the association of flavins with fatty acid metabolism, glucose metabolism also involves multiple enzymes with these embedded flavins, including PDHc.
Rotenone rottens Complex One activity by preventing the transfer of electrons from iron-sulfur clusters to ubiquinone. This results in electrons jamming from NADH and spilling over to oxygen via FMN [at I(F)]. Rotenone-inhibition of Complex I is known to increase the formation of ROS, but PDHc inhibition might produce them at a much higher rate:
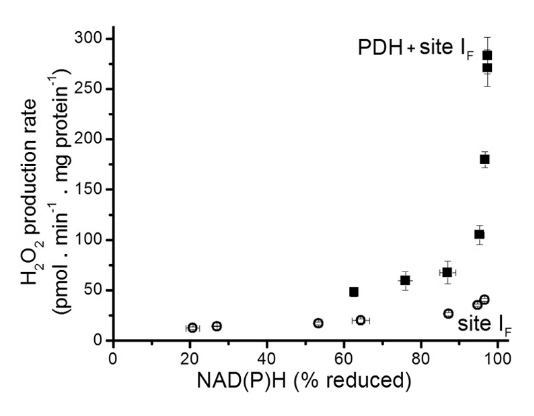
⠀(10.1074/jbc.M113.545301)
However, to maximize the production of reactive species for destabilizing purposes, knowing that KGDHc is a greater source of these species than PDHc, we can expect that fatty acid catabolism (whose decarboxylations occur entirely in the TCA cycle) is more destabilizing than the catabolism of glucose. Overexposure to fatty acids creates a challenge for the cell: exaggerated catabolism would be stressful, but their deposition in lipid droplets could eventually disturb the cell as well.
Meanwhile, pyruvate and lactate are in equilibrium, so lowering pyruvate by promoting PDHc activity should also lower lactate as a consequence, and too much lactate is a problem on its own.
Reactive oxygen species produced within a ketoacid dehydrogenase complex can involve all catalytic subunits:

⠀(10.1074/jbc.M113.545301)
Overproduction of ROS is a good reason for normal cells to inhibit substrate consumption in a controlling attempt. When ROS levels rise, glutathione gets oxidized. Once oxidized, it can attach to lipoyl groups (glutathionylation) of E2, disconnecting E1 from E3 by rendering these transferring molecules temporarily unreactive, protecting E1 from irreversible oxidation. When stressors get under control or the body has enough antioxidant defenses, glutathione can be released and PDHc may resume activity.
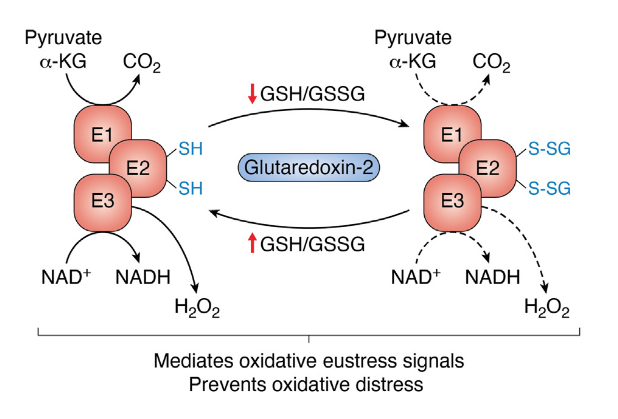
⠀(10.1016/j.jbc.2023.105399)
Local ROS levels can then regulate ketoacid dehydrogenase complexes activity without depending on kinases and phosphatases. Overwhelming exposure to ROS can lead to adverse reactions and permanent inactivation of the subunits affected.
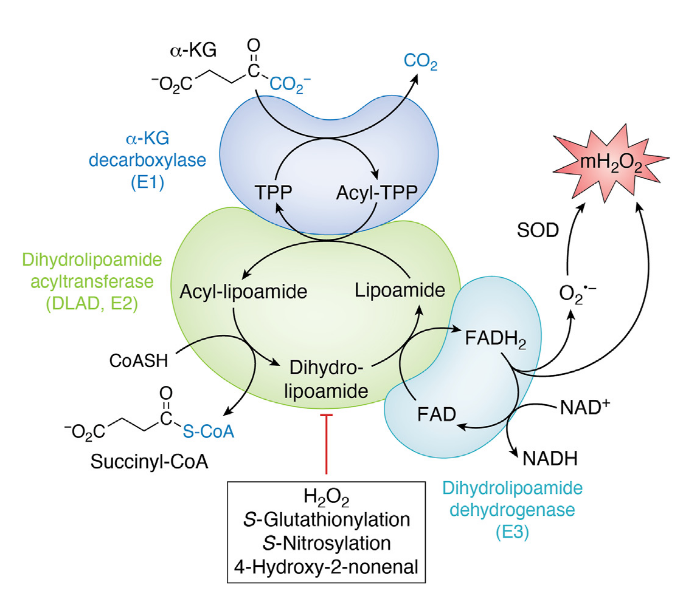
⠀(10.1016/j.jbc.2023.105399)
- 4-hydroxy-2-nonenal (HNE) is a major lipid peroxidation product.
Relevant thiols susceptible to reactive species attack:
- GSH
- CoASH
- E3cysSH
- LipoylSH
On nitrosylation, for nitric oxide (NO) donors to leave lipoic acid nonfunctional, it has to occur in the reduced, dithiol form:
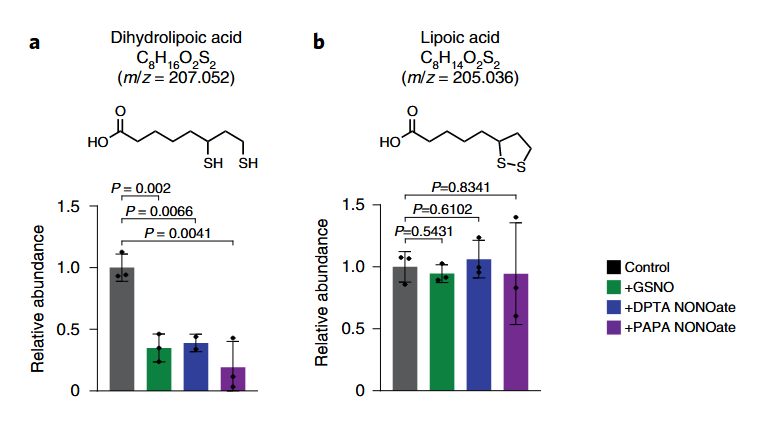
⠀(10.1038/s41589-022-01153-w)
The added compounds are NO donors. Bound (dihydro)lipoic acid is similarly affected.
The first thiol group is created with reductive acetylation from decarboxylated pyruvate and the second in transferring the acetyl group to CoA (in greater detail later).
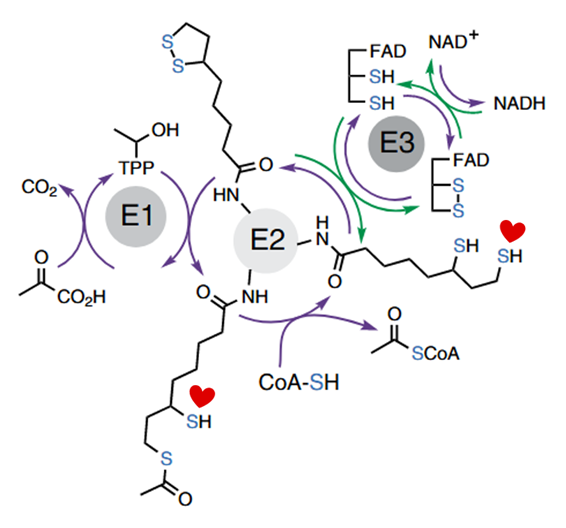
The addition of pyruvate with CoA causes lipoyl groups to occur in their susceptible, dithiol form. In this condition, NO donors become quite problematic, almost abolishing PDHc activity. But pyruvate without CoA is nowhere near as inhibitory:
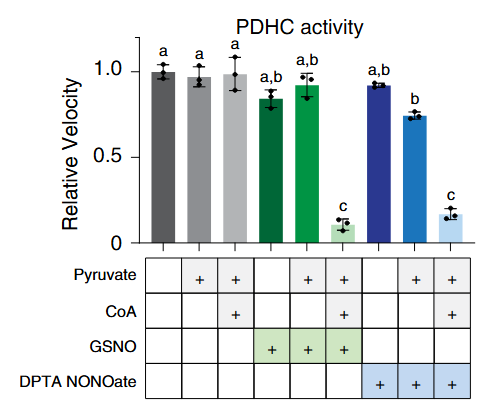
Despite the resistance of oxidized lipoic acid against NO donors, the addition of NAD⁺ (that favors oxidized lipoyl groups of PDHc) was only mildly protective:
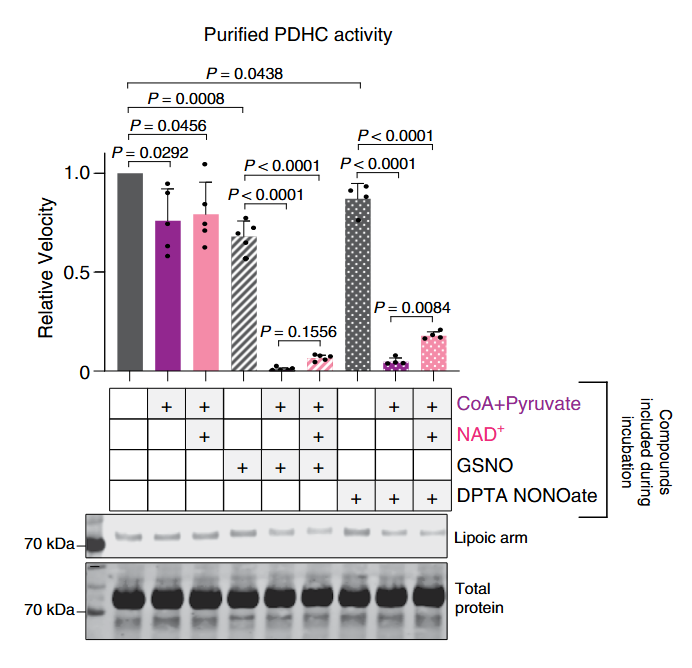
Pyruvate is the electrons donor in the forward function (purple arrows of the scheme above), but NADH can also be a donor in reverse function (green arrows), and both can change the lipoyl groups to the dithiol form.
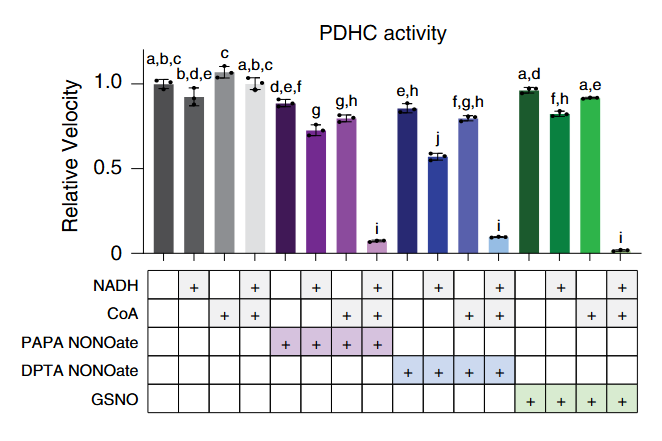
That the presence of CoA is also critical in reverse function suggests that it's behaving beyond an acetyl group acceptor. Indeed, RNS target part of the available CoA molecules, that then proceed to modify the reduced lipoyl groups.
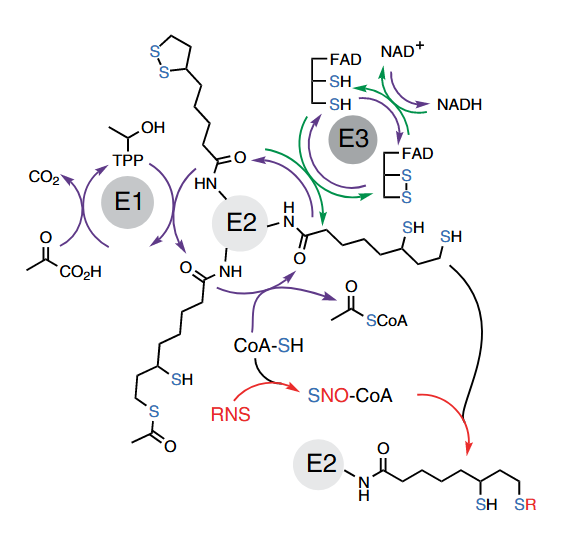
Once RNS react with CoA, intact CoA or acetyl-CoA can competitively prevent the subsequent interaction of CoA-NO with the lipoyl groups, but the amounts needed are much higher than those of CoA-NO needed to impair PDHc activity.

Therefore, in conditions of RNS overproduction, extra CoA can be problematic to the extent that it becomes an effective NO deliverer and for creating the dihydrolipoyl group that's susceptible to nitrosylation, possibly contributing to inhibit rather than stimulate PDHc.
- Arginine –iNOS→ NO + Citrulline
- Microbial pathogens → ↑LPS/IFNy → ↑iNOS → ↑NO → ↑RNS → ↑CoA-NO → ↓PDHc
- PDHc: the complex has various E1 enzymes
-
For more factors that affect PDHc, an overview of its reactions is useful:
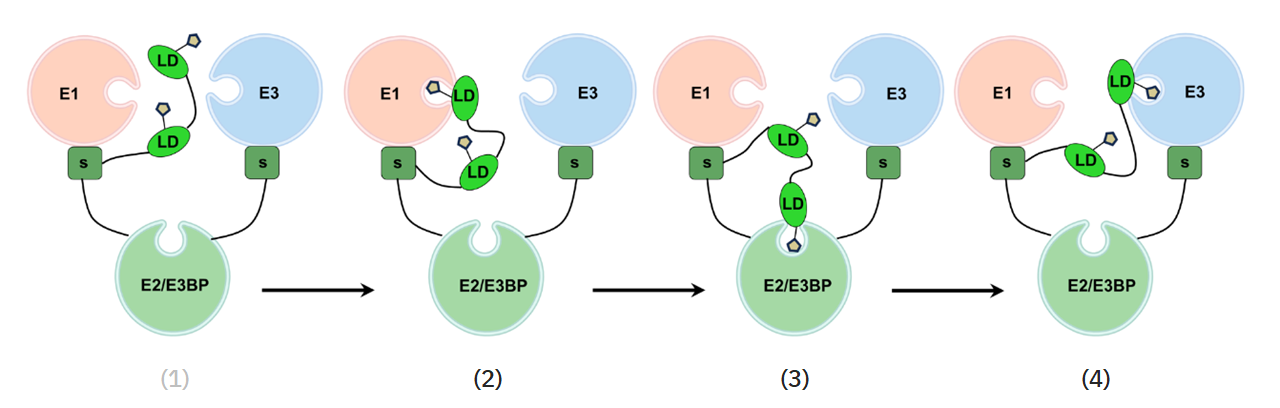
⠀(10.1038/s41467-025-56171-8)

⠀(978-0716743392)
# At Consumed Produced 1 E1 Pyruvate + H⁺ + TPP → CO₂ + HE-TPP 2 E1 HE-TPP + lipoamide ⇄ TPP + acetyl-dihydrolipoamide 3 E2(C) acetyl-dihydrolipoamide + CoA ⇄ dihydrolipoamide + acetyl-CoA 4 E3 dihydrolipoamide + FAD ⇄ lipoamide + FADH₂ 5 E3 FADH₂ + NAD⁺ ⇄ FAD + NADH + H⁺ Introduced cofactors:
- TPP from thiamin (with Mg)
- LPA from lipoic acid
- CoA from pantothenic acid
- FAD from riboflavin
- NAD from niacin
Hydroxyethyl–thiamin diphosphate (HE-TPP) is one of the post-decarboxylation intermediates.
Toxin Formula Pyruvate C₂H₃O(CO₂)⁻ Hydroxyethyl group C₂H₄O(-R) Acetyl group C₂H₃O(-R) But additional reactions take place in E1, before pyruvate binding and until the acetyl group abstraction:
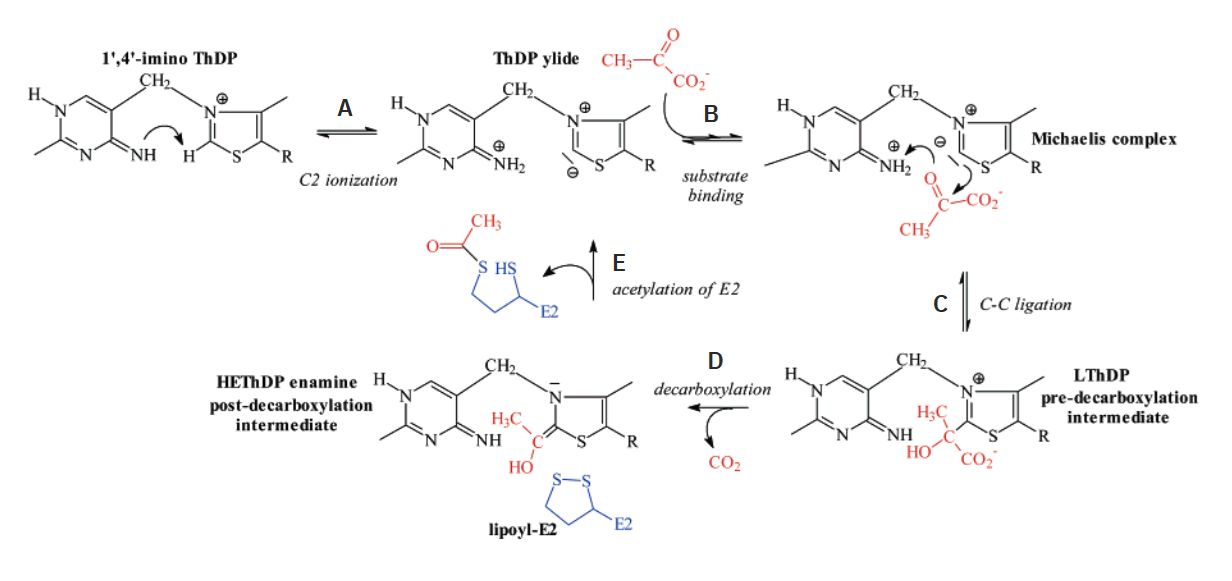
⠀(10.1021/bi700083z)
The 'rate-limiting' step appears to be the formation of 'LThDP' (C) rather than decarboxylation (D) or reductive acetylation of a lipoyl domain of E2 (E). Neat coincidence in reordering these steps with letters.
It's common for authors not to take protons (H⁺) into account in reactions. The figure just featured is one of the few that considered it:
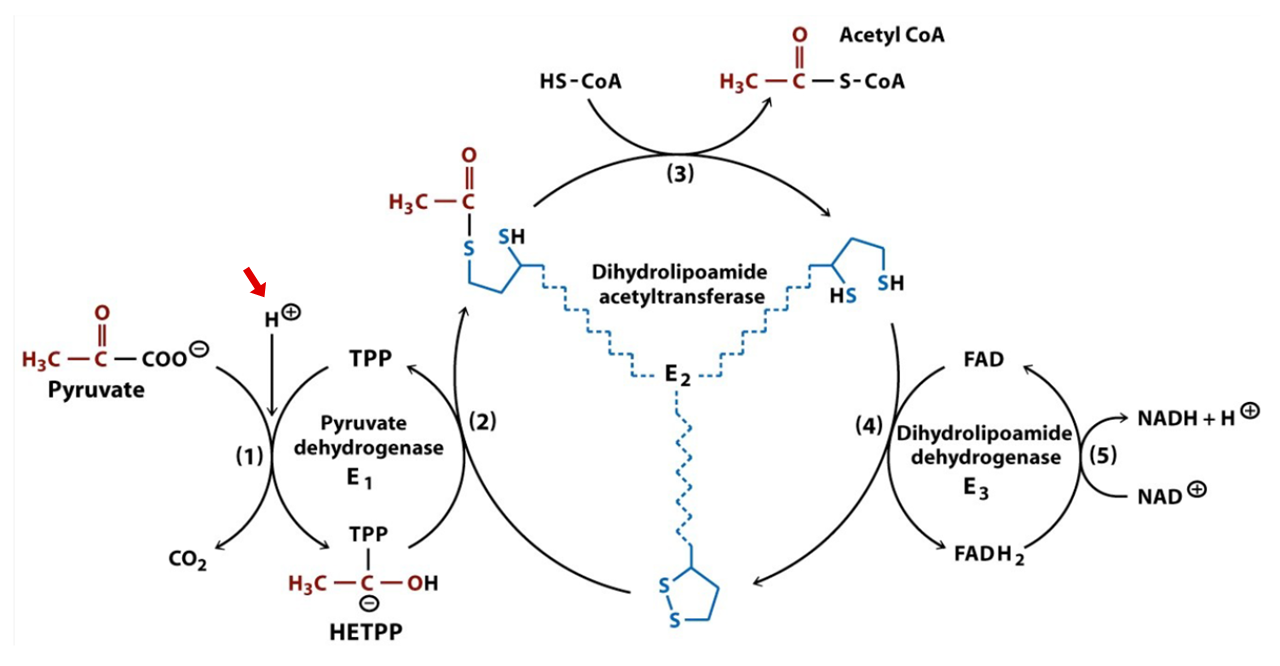
⠀(978-0716743392)
Didi and friends speculate that H⁺ move along with carboxylates (as carboxylic acids) through associated complexes and dependent reactions. Their speculation legitimizes the use of terms such as 'ketoacid or oxoacid dehydrogenases'.
The model below shows LDH associated with MCTs, which then channel a pyruvic acid equivalent directly into PDHc, suggesting that the movement of H⁺ is not random, making it seem that the H⁺ that ends up on reductively-acetylated lipoamide originates from lactate oxidation in recovering pyruvate, leaving behind NADH without H⁺:

⠀(10.3389/fnins.2018.00404)
Shuttles aside, to import NADH into mitochondria, they propose that the accompanying, but now missing, H⁺ might be sourced from hydrated CO₂, conveniently released by PDHc:
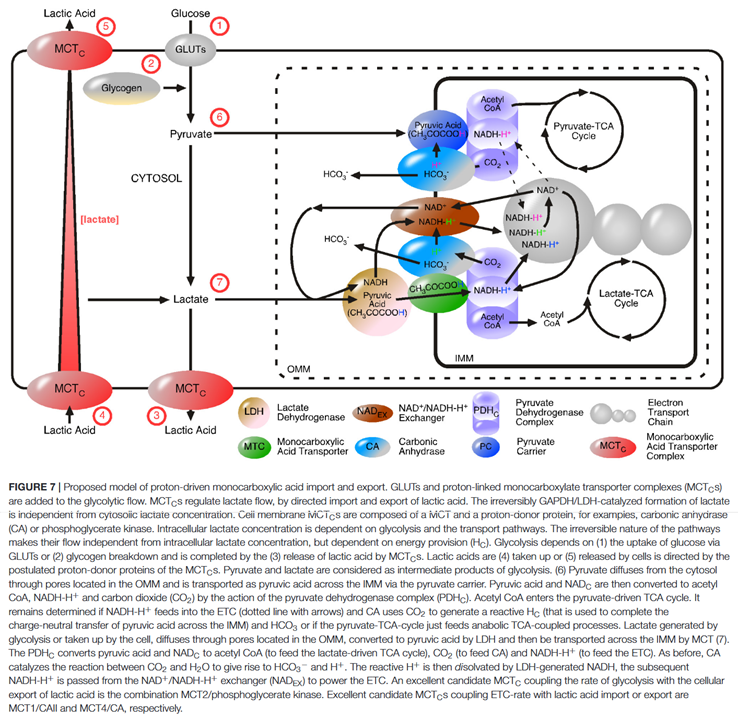
⠀(10.3389/fnins.2018.00404)
On a related note, PDHc seems to concentrate close to the interface of mitochondria, on the matrix side. This strategic location would put it in a favored position to metabolize pyruvate as it gets imported from the cytosol.
If you return to the image detailing what happens in E1, the first step indicates how bound TPP must deprotonate to become active (step A: 'C2 ionization').
A proposed principle for PDHc activity involves the two catalytic sites of E1 being connected and interacting through a tunnel lined by various acidic amino acid residues.
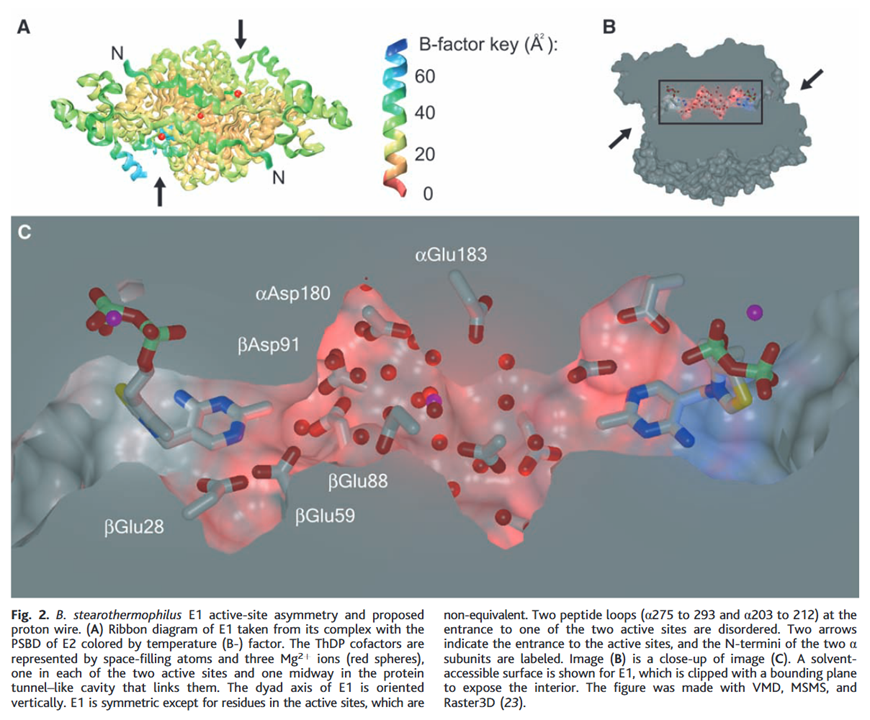
⠀(10.1126/science.1101030)
The yellow of sulfur makes it easy to spot TPP on each end of the tunnel.
In this model, protons migrate through amino acid residues within the tunnel while remaining confined to it. Deprotonation of TPP on one end would follow a shift in proton movement towards the opposite side. This redistribution would eventually impact the other end, preventing deprotonation of its TPP.
Where TPP deprotonates (−H⁺) and becomes active, the binding of pyruvate would soon make the pyruvate-TPP complex temporarily reuptake a proton released in activation; the rest of protons will follow and stream back, allowing deprotonation and activation of the opposite side.
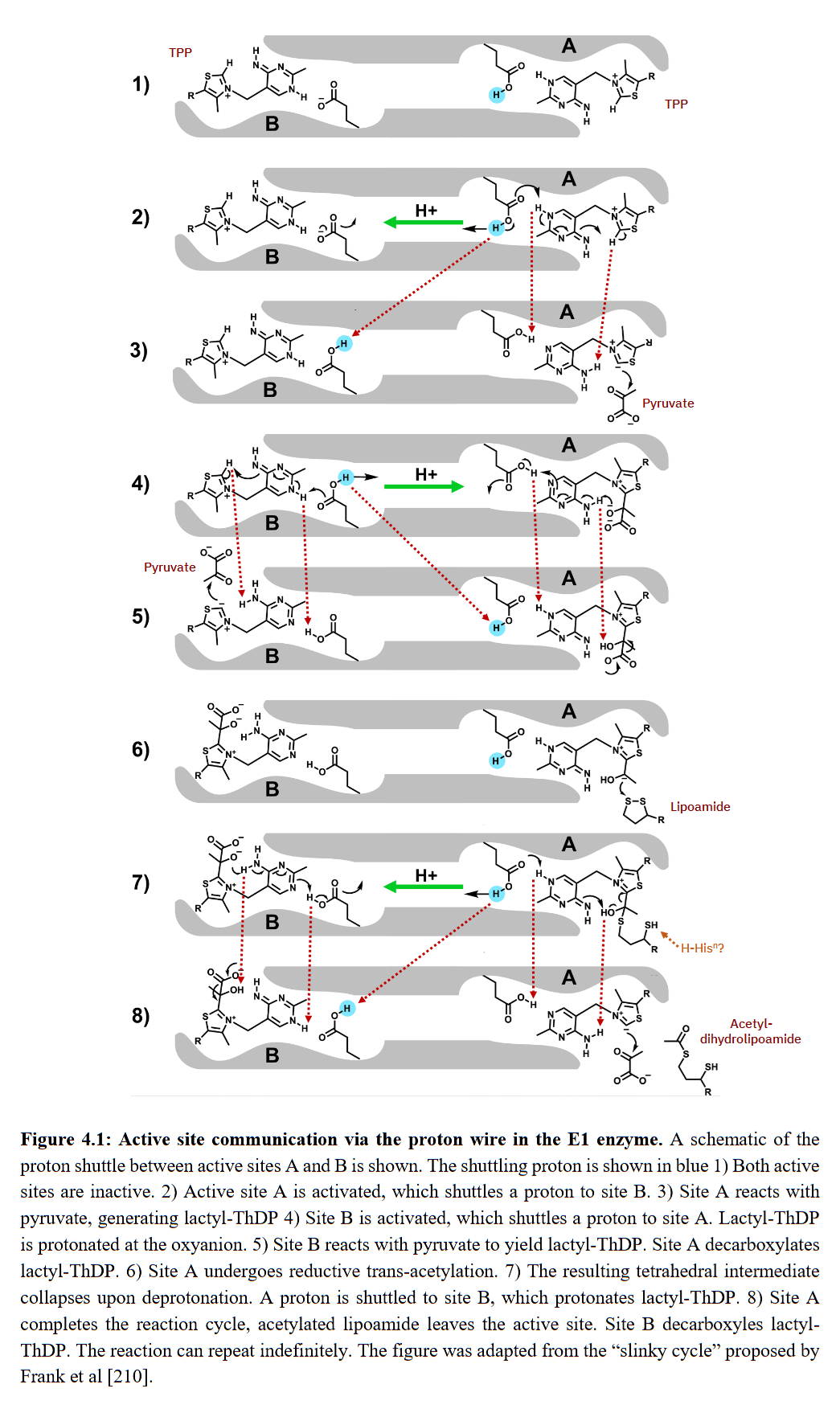
⠀(10.53846/goediss-9875)
On step 7, the hydroxyl group (-OH or HO-) still occurs in the post-decarboxylation intermediate by the time that the thiol group (-SH) already appeared in lipoamide. The immediate proton donor for this thiol group seems to be another amino acid residue where the tunnel widens, possibly histidine.
If an amino acid residue donates a H⁺ to lipoamide, for it to become a donor again for next reactions, a H⁺ must be replenished, and a way to replenish without depleting the internal pool would be from an external source. It's not clear to me the origin of this external H⁺ and whether histidine functions as a H⁺ sequestrant until the reaction is complete, but it's not as straightforward as those authors painted with pyruvic acid.
Regardless of how protons are replenished, we have a cycle: TPPs on each extremity alternate between proton donor and acceptor, switching between active and inactive forms. TPPs get reused in this model of alternating active-site reactivity.
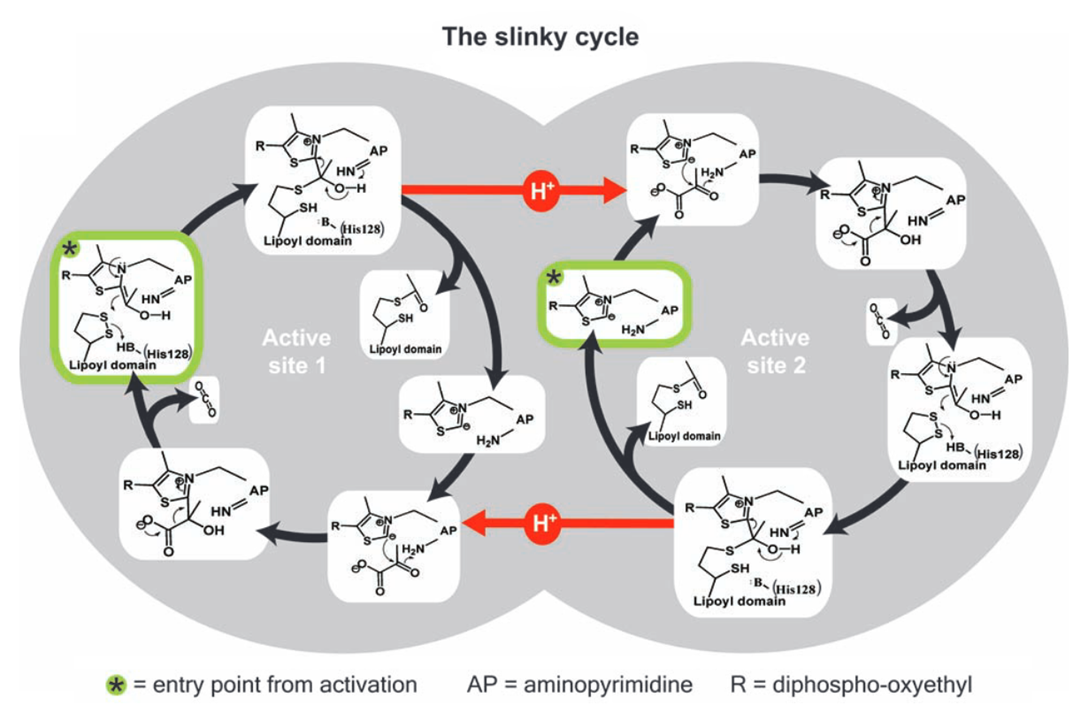
⠀(10.1126/science.1101030)
Protons redistribute following pyruvate binding.
Throughout this text, 'lipoyl domain' refers to the domain with the attached lipoyl group (holo form).
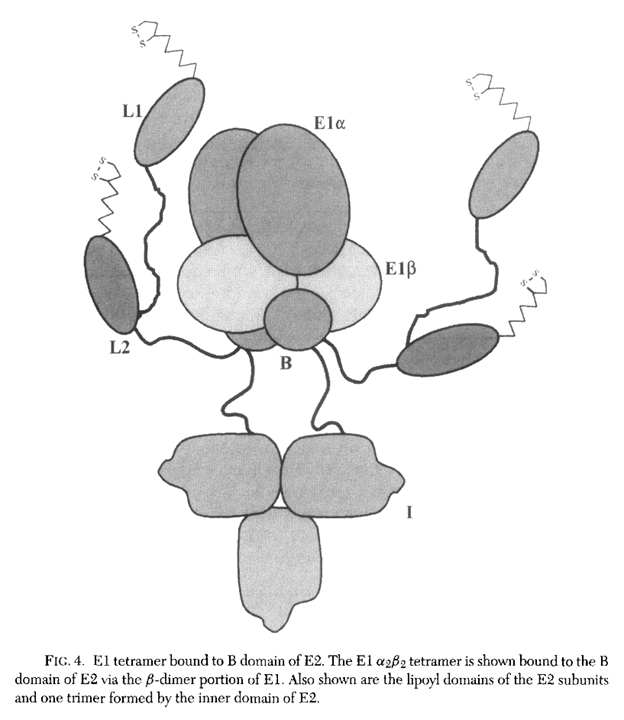
⠀(978-0-12-540070-1)
But the lipoyl domain can also occur without the attached lipoyl group (apo form).
As a side note, sirtuin 4 can hydrolyze the lipoyl groups from such domains, and this delipoylase activity is more efficient than its expected deacetylase one.
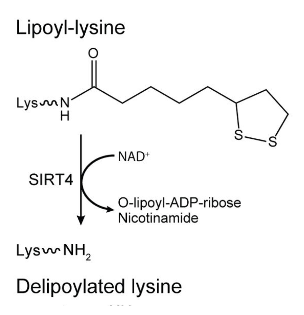
⠀(10.1016/j.cell.2014.11.046)
Lipoic acid is derived from caprylic acid (8-carbon saturated fatty acid), sometimes referred to as octanoic or goatic acid. To produce caprylic acid, the body relies on standard cycles of fatty acid synthesis, but instead of building up to 16 carbons for palmitic acid as usual, the process stops earlier with less cycles, when the molecule reaches 8 carbons.
Fatty acid synthesis occurs in the cytosol, but it's possible for it to take place in mitochondria as well. If any legume is reading, the information should interest you:
- Why do mitochondria synthesize fatty acids? Evidence for involvement in lipoic acid production
- Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology
Fatty acids are synthesized while bound to a carrier protein, having their carboxylic acid end slightly modified for binding, resulting in fatty acyls. Once the body achieves the desired chain length, the resulting octanoyl is transferred to a lysine residue of a base protein (7).
Then, with the introduction of sulfur atoms from iron-sulfur clusters to octanoyl (8), the thiol groups are created. The derived molecule is lipoamide, which is a combination of lipoic acid (also known as thioctic acid) attached to a lysine residue: lipoyl + lysyl groups.
Lastly, the lipoyl group is transferred to the target protein, which would be the lipoyl domains of E2 ('DLAT') in this case (9).
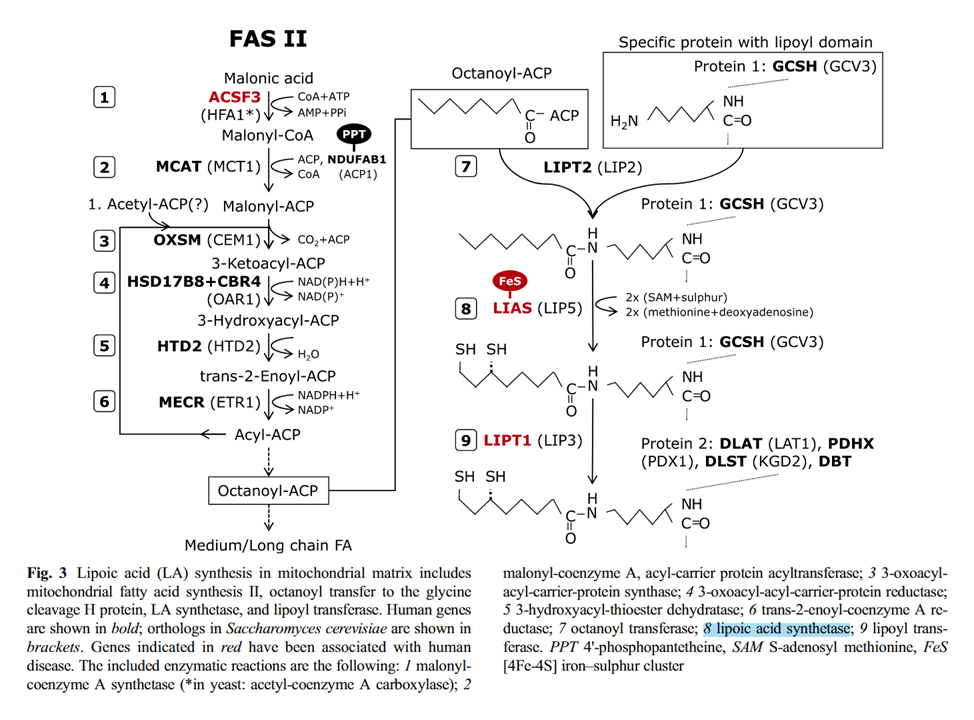
⠀(10.1007/s10545-014-9705-8)
Fatty acid synthesis (left) Lipoamide synthesis from octanoyl (right) MgATP, HCO₃⁻, B7, B3 MgATP, SAM, Fe-S (sulfur donor), B2 In addition to the ketoacid dehydrogenase complexes, the lipoyl domains are also present in the glycine cleavage system (GDHc or GCS). Note the similarities between pyruvate and glycine catabolism:
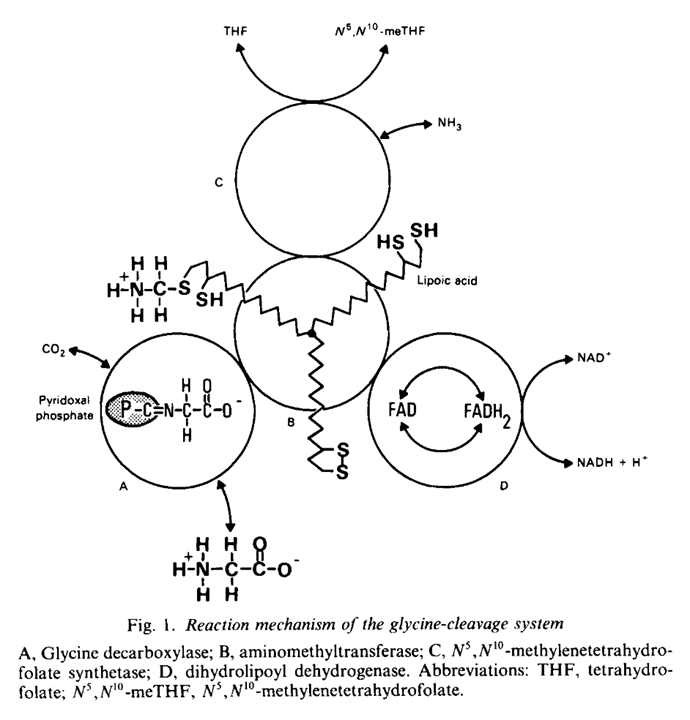
⠀(10.1042/bst0141004)
As with pyruvate, glycine ('aminoacetate') is first decarboxylated relying on
TPPPLP, then transfered to a lipoyl domain, deaminated and the2-carbon1-carbon molecule accepted byCoATHF.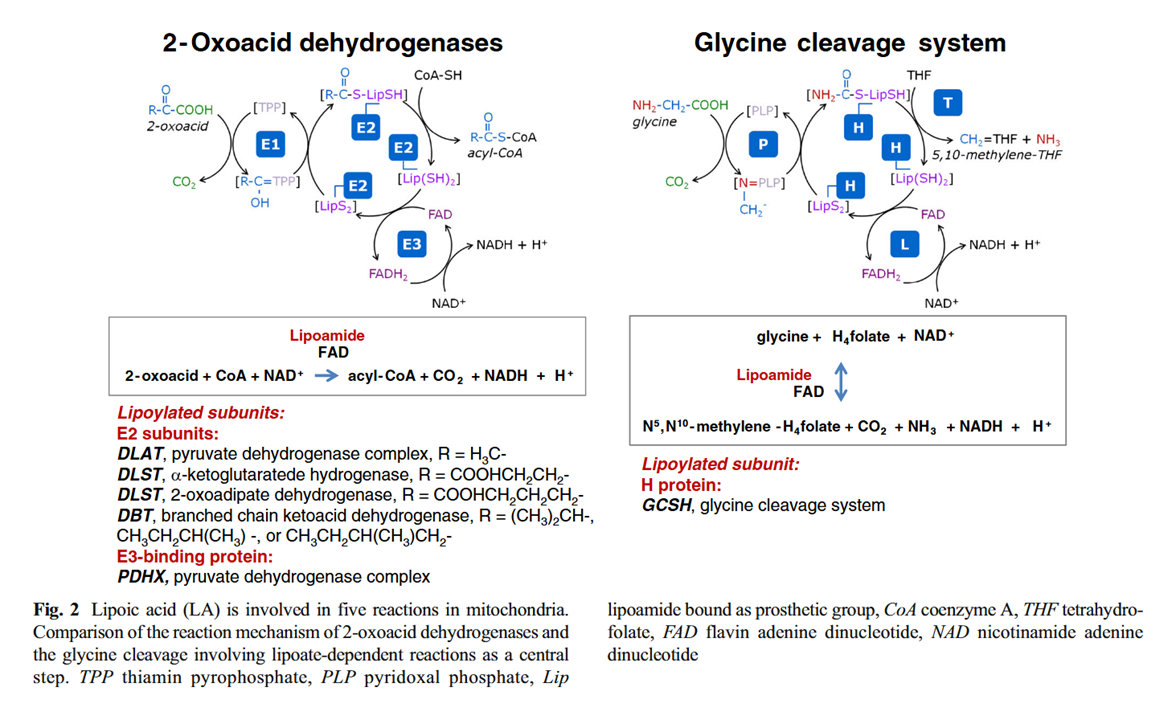
⠀(10.1007/s10545-014-9705-8)
The overall forward reaction of PDHc..
Consumes:
- Pyruvate + H⁺ + CoASH + NAD⁺
Produces:
- CO₂ + Acetyl-SCoA + NADH + H⁺
We expect the products to later..
- Consume: MgADP + Pi (+ H⁺) [or: AMP + 2Pi + Mg]
- Produce: MgATP (+ H₂O)
The general response of PDHc:
- Substrates abundance and/or energy deficit → ↑PDHc
- Products abundance and/or energy excess → ↓PDHc
The phosphate group used by PDK for inactivation of PDHc is derived from ATP in a reaction that yields ADP and a proton, which is released from the hydroxyl group of serine.
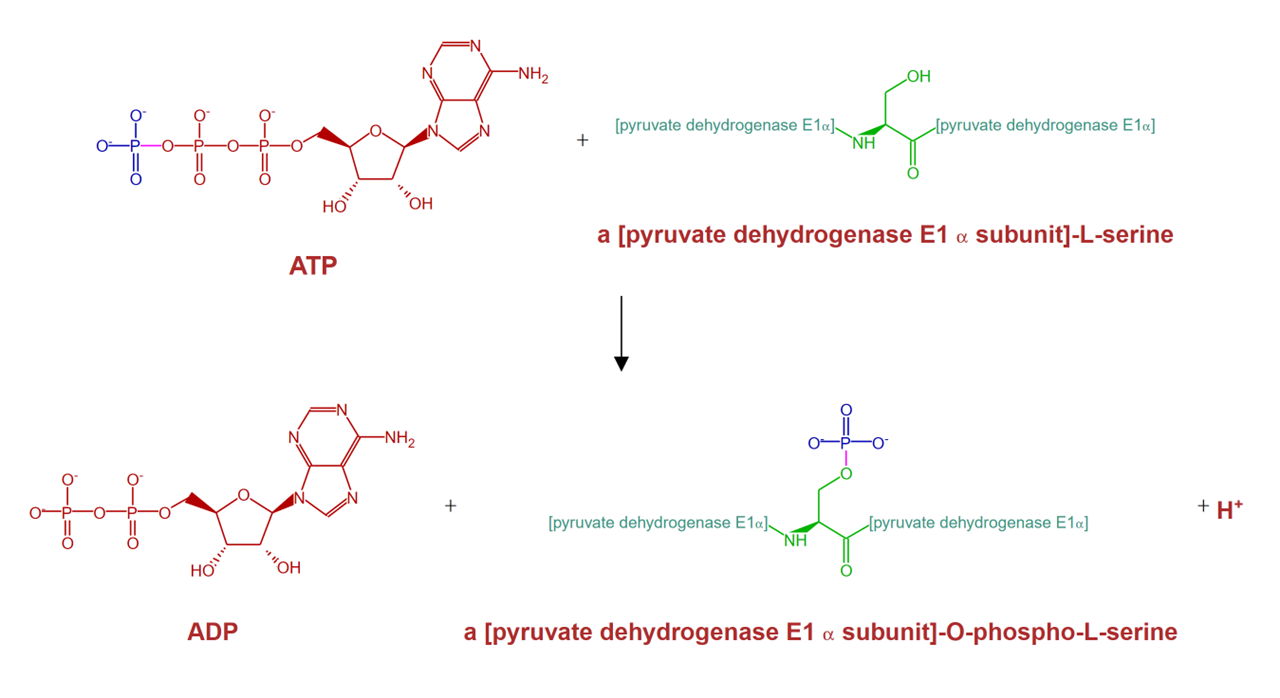
⠀(source:
the internetMetaCyc)When the products of PDHc are present in excess, either from insufficient recycling to their original forms or from being generated in conflicting amounts via other pathways (β-oxidation, ketolysis, 'amino acidolysis'), PDHc reactions can be partially reversed:
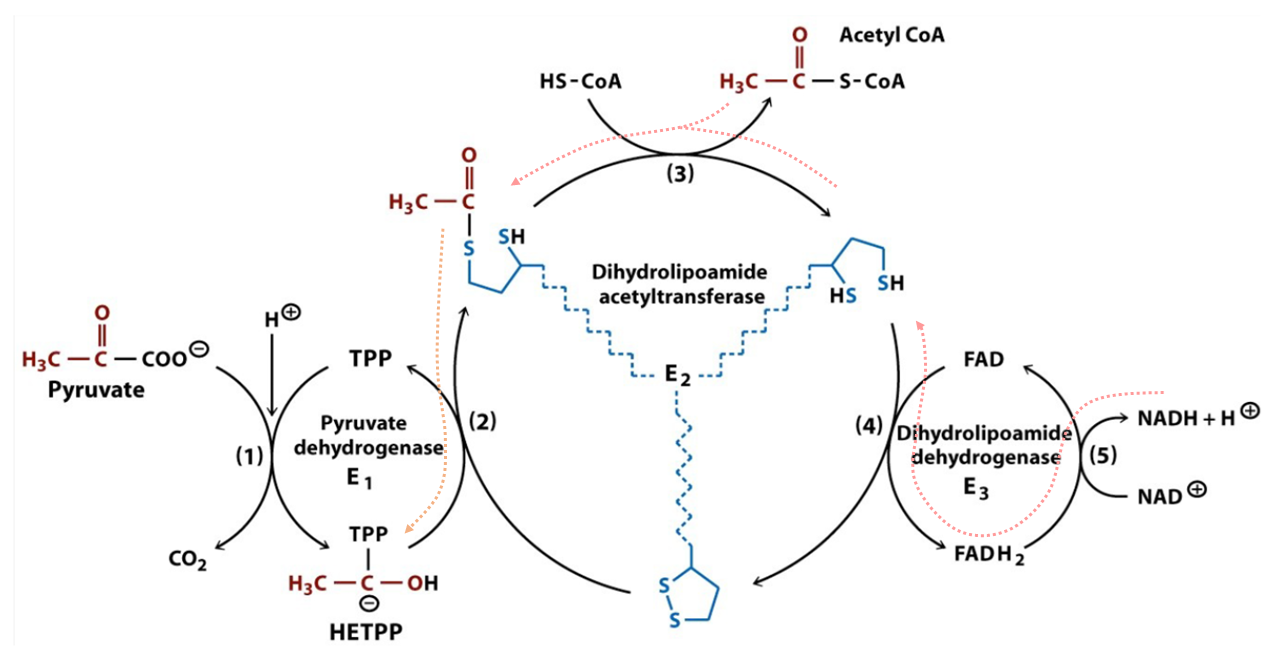
⠀(978-0716743392)
# At ConsumedProducedProducedConsumed5 E3 FADH₂ + NAD⁺ ↶ FAD + NADH + H⁺ 4 E3 dihydrolipoamide + FAD ↶ lipoamide + FADH₂ 3 E2(C) acetyl-dihydrolipoamide + CoA ↶ dihydrolipoamide + acetyl-CoA 2 E1 HE-TPP + lipoamide ↶ TPP + acetyl-dihydrolipoamide 1E1Pyruvate + H⁺ + TPP
CO₂ + HE-TPP In experiments, they usually test PDHc inhibition in additive manner, starting with NADH (↶#5) and compounding the effect with acetyl-CoA (↶#3), for the reductive + acetylation.
HE-TPP can be formed in either direction:
- Forward PDHc: Pyruvate → HE-TPP
→ acetyl-dihydrolipoamide - Backward PDHc:
Pyruvate ↚HE-TPP ← acetyl-dihydrolipoamide
Reductive acetylation of TPP to HE-TPP would impair PDHc in a direct manner, but the regulation by PDKs is more sophisticated than this. Partial modification of the lipoyl domains in reverse operation can be enough to induce the few PDKs available to spread the effect through a series of phosphorylations, disordering E1s, preventing lipoyl domains from binding to them, and leaving PDHc unable to continue with pyruvate oxidation. PDKs don't depend on saturazione:
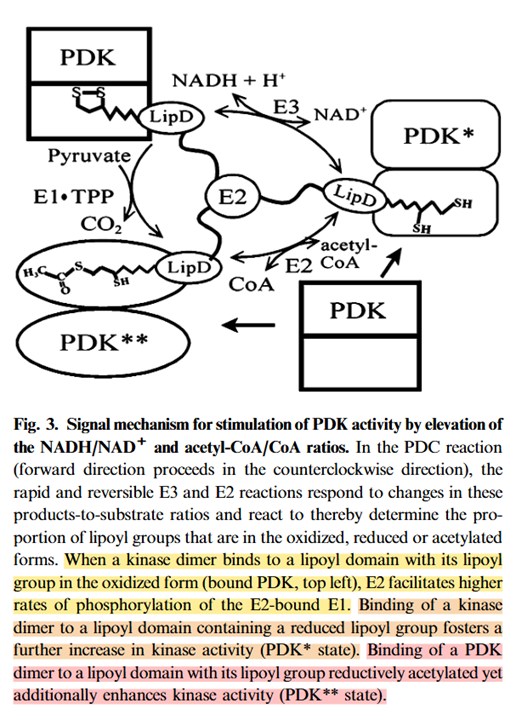
⠀(10.1046/j.1432-1033.2003.03468.x)
"Half-maximal stimulation of the activity of bovine kidney kinase is reached when <10% of the lipoyl groups in the assembled complex are acetylated. Near-maximal stimulation of the kinase activity is attained with an NADH/NAD+ ratio of 0.1 and acetyl-CoA/CoA ratio of 0.2."
The PDKs and PDPs are effective despite their low count per PDHc, but they are not always bound to the lipoyl domains of E2 or E3BP. They can occur in free form as well, and their numbers can increase without depending on available lipoyl domains. An example is PDK4, which is highly-inducible when fatty acids prevail. Interestingly, researchers managed to fully phosphorylate equivalent target sites with it, so the halving of phosphorylation capacity wasn't applicable to PDK4 in free form:
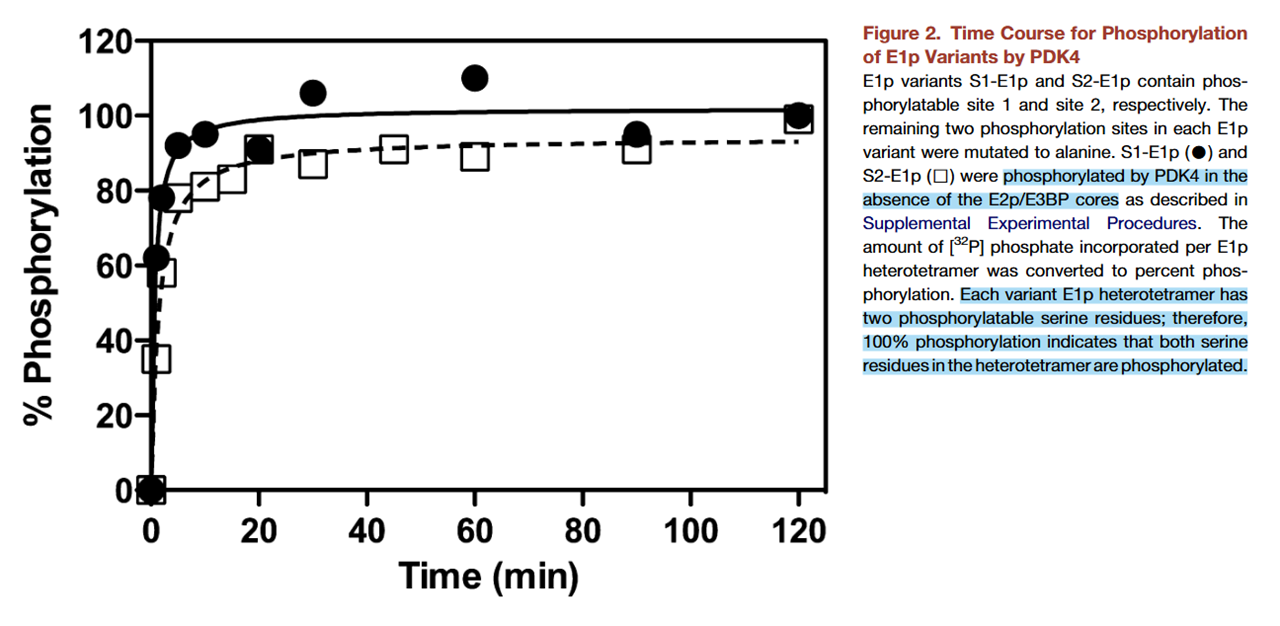
⠀(10.1016/j.str.2008.10.010)
The contribution of PDK4 to the inhibition of PDHc in the body could then be underestimated, and its occurrence might result in phosphorylation of 4 out of 6 sites.
If E1 orients in a way that favors interactions on one of its catalytic sides, it's possible for free PDK4 to have an advantage in accessing the disfavored side due to its independence from binding. However, the lack of binding and guidance may also make free PDK4 less effective at interacting with the favored side of E1, a limitation that could be compensated for by an increase in PDK4 numbers. Nevertheless, free and bound PDK4 may coexist and have a complementary action, especially considering that E1 can occur in free form as well, and PDK4 happens to have high activity towards it.
-
Now let's create a circle with candles, chant in pure bioenergetic devotion, and wait for the thiamin powder to manifest at the center.
As we snort the powder and the concentration of TPP increases, E1 (and PDHc) should get protected against the spirits of phosphorylation that inhibit its activity:
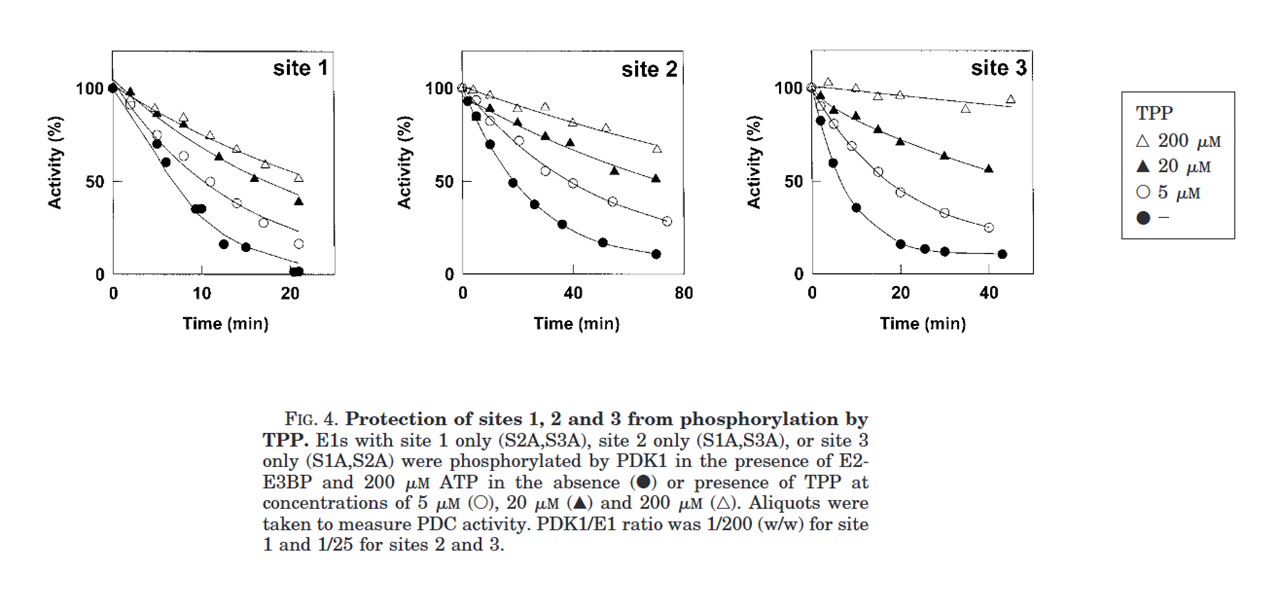
⠀(10.1074/jbc.M007558200)
Of relevance:
- 2000 μM TPP: concentration reported to saturate E1
- ~30 μM TPP: intracellular estimate (may not apply to bioenergetic evangelists)
You'll find in the graphs below that the presence of bioenergetic TPP tends to decrease the rate and degree of phosphorylation. For sites tested nearly in isolation, PDK1 and PDK2 were apparently more compromised by TPP than PDK3 and PDK4.

⠀(10.1042/bj3580069)
(While site 3 is available for phosphorylation, only PDK1 can target it. Therefore, site 3 can be ignored with other PDK isoforms; for PDK2–4, the circles and squares represent sites 1 and 2.)
Whether TPP was added or not, observe how the rate of phosphorylation by PDKs is generally higher for site 1 than 2, which in turn is higher than site 3.
- Site 1 > Site 2 > Site 3
Significance: none. I'm producing the humors. Since phosphorylation of a single site is enough for a near-complete inactivation of PDHc, targeting of site 1 suffices for immediate adaptations, with PDK2 being its major targeter. PDK2 is the 'ubiquitous' isoform.
The results are in line with the earlier table, that can be expanded this way:
PDK2 PDK3 PDK4 PDK1 Site 1 



Site 2 


Site 3 - - - 
All PDKs can phosphorylate sites 1 and 2, but PDK2 is usually limited to site 1, and only PDK1 can phosphorylate site 3. Other isoforms may be present with PDK2 in tissues, and most of them phosphorylate site 2 at a higher rate than PDK2.
It's a nuisance that the PDK isoforms were numbered in order of cloning rather than on a more meaningful basis, such as systemic relevance, phosphorylation capacity, or Kvothe's luck numbers. Reordering PDKs for systemic relevance, we would probably have:
- PDK2 > PDK4 > PDK1 > PDK3
Moving on from tests that isolate phosphorylation sites, this is the unrestricted behavior of PDKs shown earlier, but now compared to the addition of bioenergetic TPP:
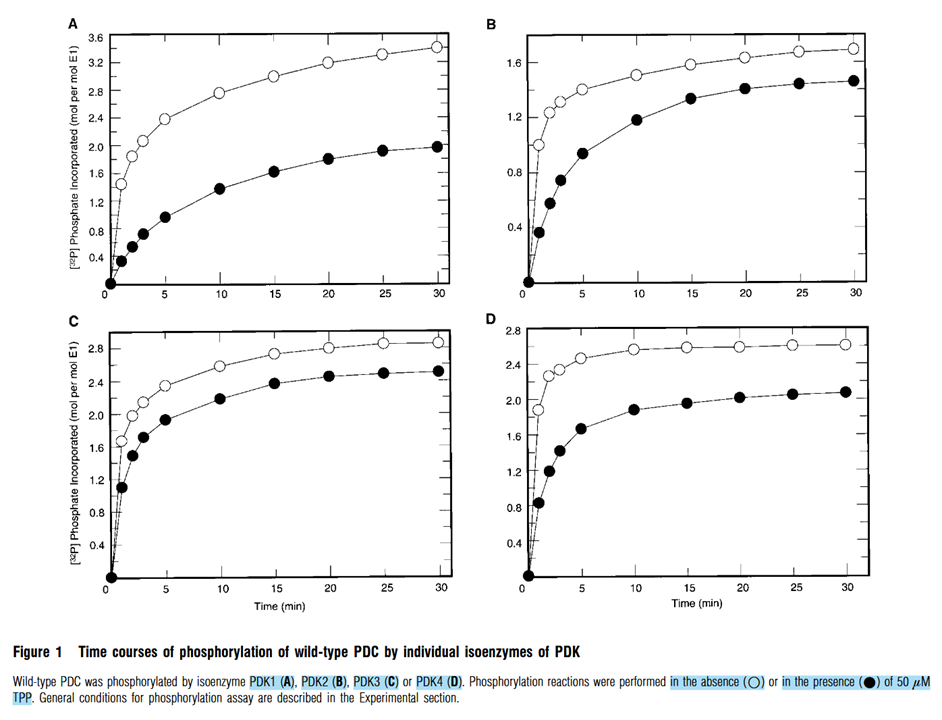
The presence of TPP inhibits phosphorylation through all PDK isoforms, but they're susceptible in this order:
- PDK1 > PDK4 > PDK2 > PDK3.
Making PDK3 the least responsive to thiamin.
As far as I know, thiamin affects the kinases, but not the phosphatases. Therefore, bioenergetic thiamin might not reactivate PDHc that's already inhibited. But it can help by gradually preventing the renewal of inhibition in further phosphorylation. Inhibiting and inhibitor should lead to disinhibition of PDHc over time.
Similar to DCA, repeated thiamin dosing must help to sustain this antiphosphorylating effect, taking advantage of the slower decay of intracellular relative to extracellular TPP levels.
Some authors report that pyruvate decarboxylation, the irreversible reaction, could still occur in saturating TPP conditions despite E1 phosphorylation, which is unexpected considering that phosphorylation compromises TPP and lipoyl domains binding. The reaction would take place in a disordered and inhibited state:
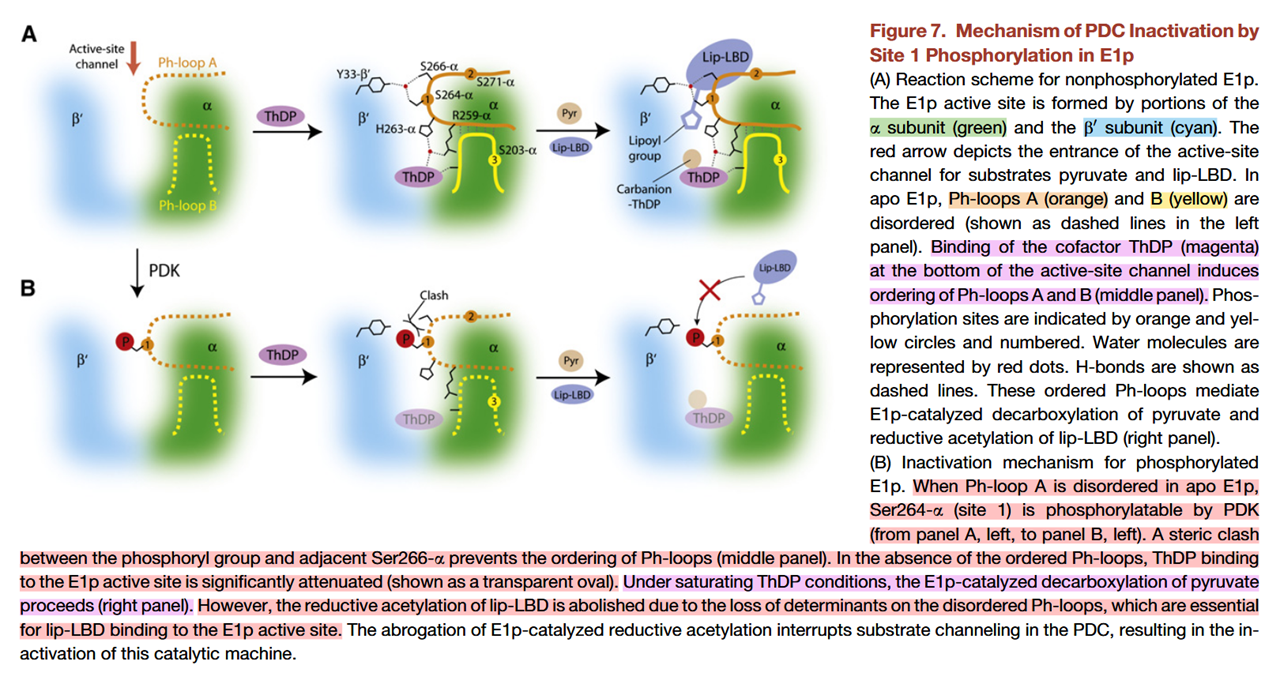
⠀(10.1016/j.str.2008.10.010)
-
When it comes to dephosphorylation of individual sites, PDP1 and PDP2 have higher activity towards site 2 > site 3 > site 1.
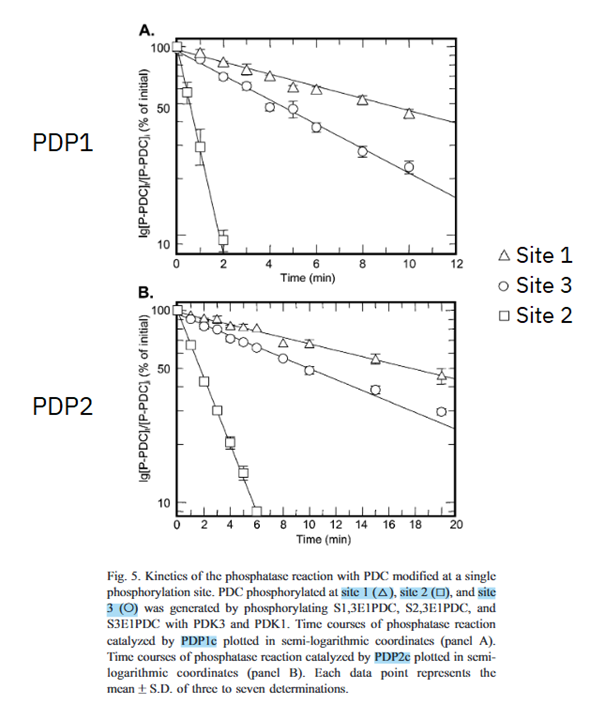
⠀(10.1016/j.bbapap.2003.08.010)
However, when PDHc is phosphorylated at site 3, site 2 is likely already occupied, whose occupation in turn likely occurred after site 1. Since tissues often have a mixture of PDKs, the action of different PDK isoforms can be complementary to increase PDHc phosphorylation.
For dephosphorylation of multiple sites, PDP1 and PDP2 show clear differences in behavior. Phosphorylation of either site 2 or 3 in addition to site 1 decreases markedly the rate of PDP2 activity:
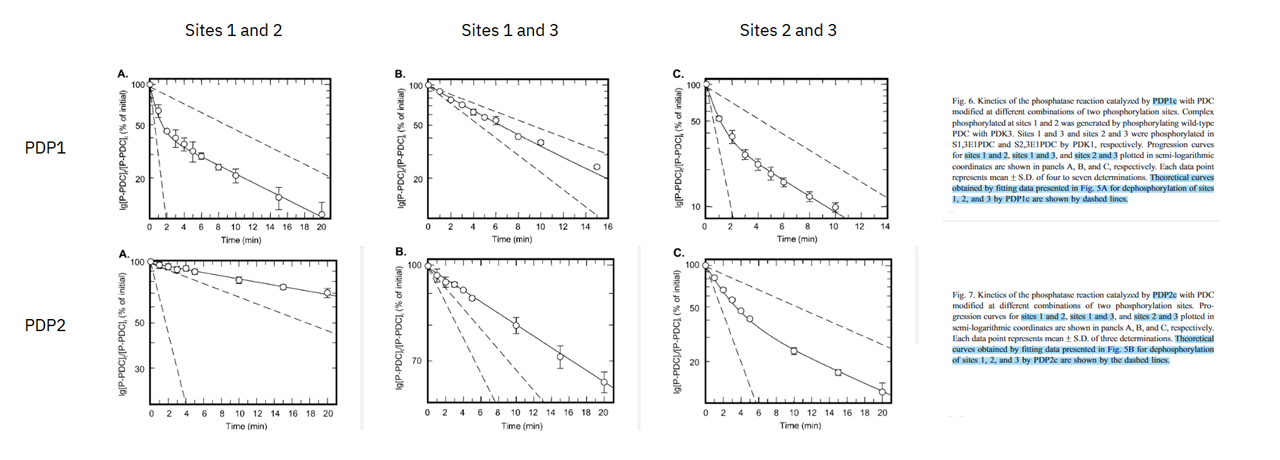
⠀(10.1016/j.bbapap.2003.08.010)
Was is inconsistent scale choice.
For the response of PDHc to dephosphorylations, the initial phosphorylation degree matches the difficulty of PDPs to reactivate it:
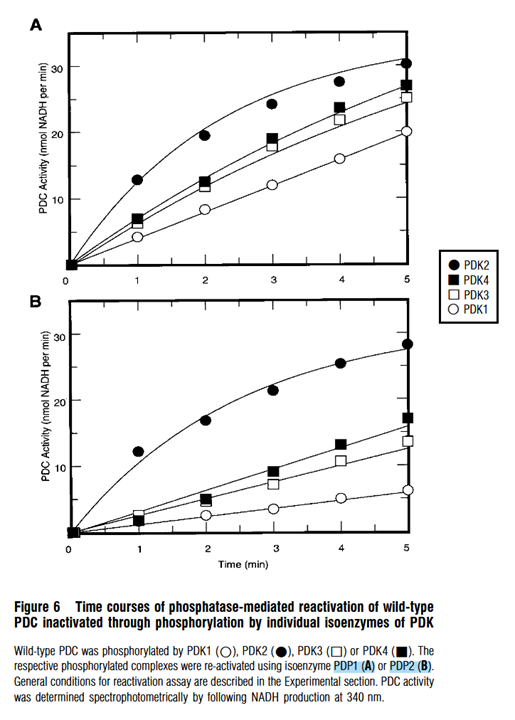
⠀(10.1042/bj3580069)
Which shows what was expected: PDHc inactivated by PDK2 (1P/E1) is the least and by PDK1 (≤3P/E1) the most resistant to reactivation, with PDK3 or PDK4 (≤2P/E1) lying somewhere in between.
PDP1 and PDP2 reactivate PDHc targeted by PDK2 (mainly S1) at an approximate rate, but PDP2 lags behind to reactivate PDHc targeted by the other PDK isoforms, reinforcing that PDP2 is disproportionately compromised when multiple sites are phosphorylated.
-
Various factors beyond the basic ones influence the regulation of PDHc by kinases (PDKs) and phosphatases (PDPs), making it more practical to put it all together into graphics to overview their behavior.
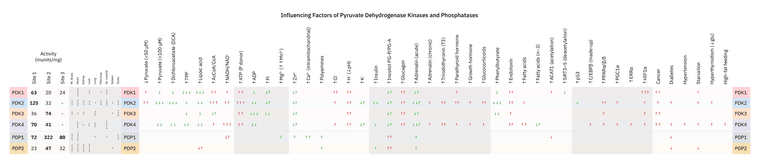
The intensity is relative to the items within the same column, less so between columns.
Color convention is tricky because what's good or bad depends on the situation. A few examples:
-
If cells glycolyze while glucose is in chronic shortage, it can be advantageous to have PDK isoforms that are less responsive to pyruvate accumulation. This way, glycolysis occurs without proceeding to irreversible mitochondrial oxidation, diverting pyruvate to lactate for export and later glucose regeneration [
acetyl-CoA ↚pyruvate –LDH→ lactate →→ glucose]. -
The same insensitivity to pyruvate may be useful in a catabolic state when excess amino acids are liberated along with fats, allowing pyruvate to accumulate and function as an another amino acceptor in (trans)amination reactions [
acetyl-CoA ↚pyruvate + amino acid –ALT→ alanine + keto acid]. Alanine is an important amino carrier and pyruvate can help to redistribute amino groups. -
Whether pyruvate passes through lactate or not, channeling it to oxaloacetate would be needed for glucose regeneration or to relieve an excess of acetyl-CoA.
Uncontrolled production of gluconeogenic precursors can contribute to the hyperglycemia of disease.
In trying to predict the response to substances, PDK and PDP must be considered together, because if both are stimulated or inhibited equally, they can negate each other. For example, potassium increases the affinity of PDK for ADP, but also ATP, so neither effect prevails.
The complexity of PDHc regulation and behavior is at odds with any faithful reliance on glorified substances. Consider the case of PDK3 with PDP2, which illustrates this point:
Even though PDK3 doesn't phosphorylate PDHc fully, it's far less sensitive to effectors than other isoforms, making PDK3 arguably more problematic than PDK1.
Someone with cancer may megadose bioenergetic thiamin and niacin intending to reactivate PDHc, not knowing that the cancer in question might express preferentially PDK3, which is relatively insensitive to thiamin and non-responsive to niacin.
- Overexpression of Pyruvate Dehydrogenase Kinase 3 Increases Drug Resistance and Early Recurrence in Colon Cancer
- Inhibition of PDK3 by artemisinin, a repurposed antimalarial drug in cancer therapy
PDK3 can become more responsive during energy shortages, but it's unclear when cancer cells experience this kind of stress. Some authors speculate that local episodes of energy abundance may occur in cancer, to the point of becoming a limiting factor for proliferation.
Moreover, the same person may be consuming plenty of glucose while also loading up on calcium, having read that it increases PDHc activity. Not only PDK3 won't respond to pyruvate or even DCA as anticipated, but if the prevalent phosphatase in the afflicted tissue is PDP2, PDHc becomes irresponsive to insulin and calcium as well.
Not satisfied with jumping to the conclusion that positive effects from supplementation of these substances would be from bioenergetic restoration of cellular respiration, the interpretation would probably assume that normalization occurred within cancer cells. It's a bizarre leap.
Yet another confounder to take into account would be the parallel modifications that compound PDHc inhibition:
- Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism
- Tyr-301 phosphorylation inhibits pyruvate dehydrogenase by blocking substrate binding and promotes the Warburg effect
Forcing pyruvate oxidation doesn't guarantee desirable effects in cancer. For instance, zinc accumulation promotes the synthesis of acetyl-CoA through PDHc, while also preventing its oxidation by inhibiting aconitase (ACO), isocitrate dehydrogenase (IDH), ketoglutarate dehydrogenase (KGDHc), succinate dehydrogenase (SDH), and cytochrome C oxidase (CcO). As a result, citrate builds up and gets exported, favoring lipid synthesis. This is a normal aspect of prostate physiology, but cancer cells can exploit such metabolic setup to their advantage.
"[..]normal prostate epithelial cells exhibit a “truncated” TCA cycle due to inhibition of aconitase [from zinc accumulation] needed for the production and secretion of a high quantity of citrate into the seminal fluid. Therefore, it is not surprising that prostate cancer relies on mitochondrial metabolism more than other tumour types."
"[..]we show that in prostate cancer, PDHc localizes in both mitochondria and nucleus. While nuclear PDHc controls the expression of Sterol regulatory element-binding transcription factor (SREBF) target genes by mediating histone acetylation, mitochondrial PDHc provides cytosolic citrate for lipid synthesis in a coordinated effort to sustain anabolism. In line with these evidence, we find that PDHA1 and the PDHc activator, Pyruvate dehydrogenase phosphatase 1 (PDP1), are frequently amplified and overexpressed at both gene and protein level in prostate tumours. Taken together, these findings demonstrate that both mitochondrial and nuclear PDHc sustain prostate tumourigenesis by controlling lipid biosynthesis thereby pointing at this complex as a novel target for cancer therapy."
Therefore, the picture is a little more complicated than apparent, multiple factors come together to make PDHc RAD, and it's more reliable to evaluate them in context.
-
-
Sources and more
Abbot, E. L., McCormack, J. G., Reynet, C., Hassall, D. G., Buchan, K. W., & Yeaman, S. J. (2005). Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells. The FEBS Journal, 272(12), 3004–3014. https://doi.org/10.1111/j.1742-4658.2005.04713.x
Abildgaard, C., Rizza, S., Christiansen, H., Schmidt, S., Dahl, C., Abdul-Al, A., Christensen, A., Filomeni, G., & Guldberg, P. (2021). Screening of metabolic modulators identifies new strategies to target metabolic reprogramming in melanoma. Scientific Reports, 11(1), 4390. https://doi.org/10.1038/s41598-021-83796-8
Anastasiou, D., Poulogiannis, G., Asara, J. M., Boxer, M. B., Jiang, J., Shen, M., Bellinger, G., Sasaki, A. T., Locasale, J. W., Auld, D. S., Thomas, C. J., Vander Heiden, M. G., & Cantley, L. C. (2011). Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science, 334(6060), 1278–1283. https://doi.org/10.1126/science.1211485
Anwar, S., DasGupta, D., Azum, N., Alfaifi, S. Y. M., Asiri, A. M., Alhumaydhi, F. A., Alsagaby, S. A., Sharaf, S. E., Shahwan, M., & Hassan, Md. I. (2022). Inhibition of PDK3 by artemisinin, a repurposed antimalarial drug in cancer therapy. Journal of Molecular Liquids, 355, 118928. https://doi.org/10.1016/j.molliq.2022.118928
Arjunan, P., Sax, M., Brunskill, A., Chandrasekhar, K., Nemeria, N., Zhang, S., Jordan, F., & Furey, W. (2006). A Thiamin-bound, Pre-decarboxylation Reaction Intermediate Analogue in the Pyruvate Dehydrogenase E1 Subunit Induces Large Scale Disorder-to-Order Transformations in the Enzyme and Reveals Novel Structural Features in the Covalently Bound Adduct. Journal of Biological Chemistry, 281(22), 15296–15303. https://doi.org/10.1074/jbc.M600656200
Arjunan, P., Wang, J., Nemeria, N. S., Reynolds, S., Brown, I., Chandrasekhar, K., Calero, G., Jordan, F., & Furey, W. (2014). Novel Binding Motif and New Flexibility Revealed by Structural Analyses of a Pyruvate Dehydrogenase-Dihydrolipoyl Acetyltransferase Subcomplex from the Escherichia coli Pyruvate Dehydrogenase Multienzyme Complex. Journal of Biological Chemistry, 289(43), 30161–30176. https://doi.org/10.1074/jbc.M114.592915
Atas, E., Oberhuber, M., & Kenner, L. (2020). The Implications of PDK1–4 on Tumor Energy Metabolism, Aggressiveness and Therapy Resistance. Frontiers in Oncology, 10, 583217. https://doi.org/10.3389/fonc.2020.583217
Atherton, H. J., Dodd, M. S., Heather, L. C., Schroeder, M. A., Griffin, J. L., Radda, G. K., Clarke, K., & Tyler, D. J. (2011). Role of Pyruvate Dehydrogenase Inhibition in the Development of Hypertrophy in the Hyperthyroid Rat Heart: A Combined Magnetic Resonance Imaging and Hyperpolarized Magnetic Resonance Spectroscopy Study. Circulation, 123(22), 2552–2561. https://doi.org/10.1161/CIRCULATIONAHA.110.011387
Baker, J. C., Yan, X., Peng, T., Kasten, S., & Roche, T. E. (2000). Marked Differences between Two Isoforms of Human Pyruvate Dehydrogenase Kinase. Journal of Biological Chemistry, 275(21), 15773–15781. https://doi.org/10.1074/jbc.M909488199
Balakrishnan, A., Nemeria, N. S., Chakraborty, S., Kakalis, L., & Jordan, F. (2012). Determination of Pre-Steady-State Rate Constants on the Escherichia coli Pyruvate Dehydrogenase Complex Reveals That Loop Movement Controls the Rate-Limiting Step. Journal of the American Chemical Society, 134(45), 18644–18655. https://doi.org/10.1021/ja3062375
Bao, H., Kasten, S. A., Yan, X., Hiromasa, Y., & Roche, T. E. (2004). Pyruvate Dehydrogenase Kinase Isoform 2 Activity Stimulated by Speeding Up the Rate of Dissociation of ADP. Biochemistry, 43(42), 13442–13451. https://doi.org/10.1021/bi0494875
Bensard, C. L., Wisidagama, D. R., Olson, K. A., Berg, J. A., Krah, N. M., Schell, J. C., Nowinski, S. M., Fogarty, S., Bott, A. J., Wei, P., Dove, K. K., Tanner, J. M., Panic, V., Cluntun, A., Lettlova, S., Earl, C. S., Namnath, D. F., Vázquez-Arreguín, K., Villanueva, C. J., … Rutter, J. (2020). Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier. Cell Metabolism, 31(2), 284-300.e7. https://doi.org/10.1016/j.cmet.2019.11.002
Bigrigg, J. K., Heigenhauser, G. J. F., Inglis, J. G., LeBlanc, P. J., & Peters, S. J. (2009). Carbohydrate refeeding after a high-fat diet rapidly reverses the adaptive increase in human skeletal muscle PDH kinase activity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 297(3), R885–R891. https://doi.org/10.1152/ajpregu.90604.2008
Bonnet, S., Archer, S. L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R., Lee, C. T., Lopaschuk, G. D., Puttagunta, L., Bonnet, S., Harry, G., Hashimoto, K., Porter, C. J., Andrade, M. A., Thebaud, B., & Michelakis, E. D. (2007). A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell, 11(1), 37–51. https://doi.org/10.1016/j.ccr.2006.10.020
Booker, S. J. (2004). Unraveling the Pathway of Lipoic Acid Biosynthesis. Chemistry & Biology, 11(1), 10–12. https://doi.org/10.1016/j.chembiol.2004.01.002
Bowker-Kinley, M. M., Davis, I. W., Wu, P., Harris, A. R., & Popov, M. K. (1998). Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochemical Journal, 329(1), 191–196. https://doi.org/10.1042/bj3290191
Brautigam, C. A., Wynn, R. M., Chuang, J. L., & Chuang, D. T. (2009). Subunit and Catalytic Component Stoichiometries of an in Vitro Reconstituted Human Pyruvate Dehydrogenase Complex. Journal of Biological Chemistry, 284(19), 13086–13098. https://doi.org/10.1074/jbc.M806563200
Brown, G. K., Otero, L. J., LeGris, M., & Brown, R. M. (n.d.). Pyruvate dehydrogenase deficiency.
Bunik, V. I., & Sievers, C. (2002). Inactivation of the 2‐oxo acid dehydrogenase complexes upon generation of intrinsic radical species. European Journal of Biochemistry, 269(20), 5004–5015. https://doi.org/10.1046/j.1432-1033.2002.03204.x
Burnham-Marusich, A. R., & Berninsone, P. M. (2012). Multiple proteins with essential mitochondrial functions have glycosylated isoforms. Mitochondrion, 12(4), 423–427. https://doi.org/10.1016/j.mito.2012.04.004
Cai, Z., Li, C.-F., Han, F., Liu, C., Zhang, A., Hsu, C.-C., Peng, D., Zhang, X., Jin, G., Rezaeian, A.-H., Wang, G., Zhang, W., Pan, B.-S., Wang, C.-Y., Wang, Y.-H., Wu, S.-Y., Yang, S.-C., Hsu, F.-C., D’Agostino, R. B., … Lin, H.-K. (2020). Phosphorylation of PDHA by AMPK Drives TCA Cycle to Promote Cancer Metastasis. Molecular Cell, 80(2), 263-278.e7. https://doi.org/10.1016/j.molcel.2020.09.018
Cairns, R. A., Papandreou, I., Sutphin, P. D., & Denko, N. C. (2007). Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proceedings of the National Academy of Sciences, 104(22), 9445–9450. https://doi.org/10.1073/pnas.0611662104
Chalifoux, O., Faerman, B., & Mailloux, R. J. (2023). Mitochondrial hydrogen peroxide production by pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in oxidative eustress and oxidative distress. Journal of Biological Chemistry, 299(12), 105399. https://doi.org/10.1016/j.jbc.2023.105399
Chen, J., Guccini, I., Di Mitri, D., Brina, D., Revandkar, A., Sarti, M., Pasquini, E., Alajati, A., Pinton, S., Losa, M., Civenni, G., Catapano, C. V., Sgrignani, J., Cavalli, A., D’Antuono, R., Asara, J. M., Morandi, A., Chiarugi, P., Crotti, S., … Alimonti, A. (2018). Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nature Genetics, 50(2), 219–228. https://doi.org/10.1038/s41588-017-0026-3
Choi, Y. W., & Lim, I. K. (2014). Sensitization of metformin-cytotoxicity by dichloroacetate via reprogramming glucose metabolism in cancer cells. Cancer Letters, 346(2), 300–308. https://doi.org/10.1016/j.canlet.2014.01.015
Choiniere, J., Wu, J., & Wang, L. (2017). Pyruvate Dehydrogenase Kinase 4 Deficiency Results in Expedited Cellular Proliferation through E2F1-Mediated Increase of Cyclins. Molecular Pharmacology, 91(3), 189–196. https://doi.org/10.1124/mol.116.106757
Ciszak, E. M., Makal, A., Hong, Y. S., Vettaikkorumakankauv, A. K., Korotchkina, L. G., & Patel, M. S. (2006). How Dihydrolipoamide Dehydrogenase-binding Protein Binds Dihydrolipoamide Dehydrogenase in the Human Pyruvate Dehydrogenase Complex. Journal of Biological Chemistry, 281(1), 648–655. https://doi.org/10.1074/jbc.M507850200
Commander, R., Wei, C., Sharma, A., Mouw, J. K., Burton, L. J., Summerbell, E., Mahboubi, D., Peterson, R. J., Konen, J., Zhou, W., Du, Y., Fu, H., Shanmugam, M., & Marcus, A. I. (2020). Subpopulation targeting of pyruvate dehydrogenase and GLUT1 decouples metabolic heterogeneity during collective cancer cell invasion. Nature Communications, 11(1), 1533. https://doi.org/10.1038/s41467-020-15219-7
Cook, K. M., Shen, H., McKelvey, K. J., Gee, H. E., & Hau, E. (2021). Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas. International Journal of Molecular Sciences, 22(14), 7265. https://doi.org/10.3390/ijms22147265
Corbet, C., Bastien, E., Draoui, N., Doix, B., Mignion, L., Jordan, B. F., Marchand, A., Vanherck, J.-C., Chaltin, P., Schakman, O., Becker, H. M., Riant, O., & Feron, O. (2018). Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nature Communications, 9(1), 1208. https://doi.org/10.1038/s41467-018-03525-0
De Mey, S., Dufait, I., Jiang, H., Corbet, C., Wang, H., Van De Gucht, M., Kerkhove, L., Law, K. L., Vandenplas, H., Gevaert, T., Feron, O., & De Ridder, M. (2020). Dichloroacetate Radiosensitizes Hypoxic Breast Cancer Cells. International Journal of Molecular Sciences, 21(24), 9367. https://doi.org/10.3390/ijms21249367
Denton, R. M., McCormack, J. G., Rutter, G. A., Burnett, P., Edgell, N. J., Moule, S. K., & Diggle, T. A. (1996). The hormonal regulation of pyruvate dehydrogenase complex. Advances in Enzyme Regulation, 36, 183–198. https://doi.org/10.1016/0065-2571(95)00020-8
Devedjiev, Y., Steussy, C. N., & Vassylyev, D. G. (2007). Crystal Structure of an Asymmetric Complex of Pyruvate Dehydrogenase Kinase 3 with Lipoyl Domain 2 and its Biological Implications. Journal of Molecular Biology, 370(3), 407–416. https://doi.org/10.1016/j.jmb.2007.04.083
Drakulic, S., Rai, J., Petersen, S. V., Golas, M. M., & Sander, B. (2018). Folding and assembly defects of pyruvate dehydrogenase deficiency-related variants in the E1α subunit of the pyruvate dehydrogenase complex. Cellular and Molecular Life Sciences, 75(16), 3009–3026. https://doi.org/10.1007/s00018-018-2775-2
Fan, J., Shan, C., Kang, H.-B., Elf, S., Xie, J., Tucker, M., Gu, T.-L., Aguiar, M., Lonning, S., Chen, H., Mohammadi, M., Britton, L.-M. P., Garcia, B. A., Alečković, M., Kang, Y., Kaluz, S., Devi, N., Van Meir, E. G., Hitosugi, T., … Chen, J. (2014). Tyr Phosphorylation of PDP1 Toggles Recruitment between ACAT1 and SIRT3 to Regulate the Pyruvate Dehydrogenase Complex. Molecular Cell, 53(4), 534–548. https://doi.org/10.1016/j.molcel.2013.12.026
Ferriero, R., Iannuzzi, C., Manco, G., & Brunetti‐Pierri, N. (2015). Differential inhibition of PDKs by phenylbutyrate and enhancement of pyruvate dehydrogenase complex activity by combination with dichloroacetate. Journal of Inherited Metabolic Disease, 38(5), 895–904. https://doi.org/10.1007/s10545-014-9808-2
Ferriero, R., Manco, G., Lamantea, E., Nusco, E., Ferrante, M. I., Sordino, P., Stacpoole, P. W., Lee, B., Zeviani, M., & Brunetti-Pierri, N. (2013). Phenylbutyrate Therapy for Pyruvate Dehydrogenase Complex Deficiency and Lactic Acidosis. Science Translational Medicine, 5(175). https://doi.org/10.1126/scitranslmed.3004986
Fisher-Wellman, K. H., Gilliam, L. A. A., Lin, C.-T., Cathey, B. L., Lark, D. S., & Darrell Neufer, P. (2013). Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radical Biology and Medicine, 65, 1201–1208. https://doi.org/10.1016/j.freeradbiomed.2013.09.008
Fitzgerald, J., Hutchison, W. M., & Dahl, H.-H. M. (n.d.). Isolation and characterisation of the mouse pyruvate dehydrogenase Ela genes.
Frank, R. A. W., Kay, C. W. M., Hirst, J., & Luisi, B. F. (2008). Off-Pathway, Oxygen-Dependent Thiamine Radical in the Krebs Cycle. Journal of the American Chemical Society, 130(5), 1662–1668. https://doi.org/10.1021/ja076468k
Frank, R. A. W., Pratap, J. V., Pei, X. Y., Perham, R. N., & Luisi, B. F. (2005). The Molecular Origins of Specificity in the Assembly of a Multienzyme Complex. Structure, 13(8), 1119–1130. https://doi.org/10.1016/j.str.2005.04.021
Frank, R. A. W., Titman, C. M., Pratap, J. V., Luisi, B. F., & Perham, R. N. (2004). A Molecular Switch and Proton Wire Synchronize the Active Sites in Thiamine Enzymes. Science, 306(5697), 872–876. https://doi.org/10.1126/science.1101030
Fries, M., Jung, H.-I., & Perham, R. N. (2003). Reaction Mechanism of the Heterotetrameric (α2β2) E1 Component of 2-Oxo Acid Dehydrogenase Multienzyme Complexes. Biochemistry, 42(23), 6996–7002. https://doi.org/10.1021/bi027397z
Geng, X., Elmadhoun, O., Peng, C., Ji, X., Hafeez, A., Liu, Z., Du, H., Rafols, J. A., & Ding, Y. (2015). Ethanol and Normobaric Oxygen: Novel Approach in Modulating Pyruvate Dehydrogenase Complex After Severe Transient and Permanent Ischemic Stroke. Stroke, 46(2), 492–499. https://doi.org/10.1161/STROKEAHA.114.006994
Gokcan, H., Bedoyan, J. K., & Isayev, O. (2022). Simulations of Pathogenic E1α Variants: Allostery and Impact on Pyruvate Dehydrogenase Complex-E1 Structure and Function. Journal of Chemical Information and Modeling, 62(14), 3463–3475. https://doi.org/10.1021/acs.jcim.2c00630
Golias, T., Kery, M., Radenkovic, S., & Papandreou, I. (2019). Microenvironmental control of glucose metabolism in tumors by regulation of pyruvate dehydrogenase. International Journal of Cancer, 144(4), 674–686. https://doi.org/10.1002/ijc.31812
Golias, T., Papandreou, I., Sun, R., Kumar, B., Brown, N. V., Swanson, B. J., Pai, R., Jaitin, D., Le, Q.-T., Teknos, T. N., & Denko, N. C. (2016). Hypoxic repression of pyruvate dehydrogenase activity is necessary for metabolic reprogramming and growth of model tumours. Scientific Reports, 6(1), 31146. https://doi.org/10.1038/srep31146
Grassian, A. R., Metallo, C. M., Coloff, J. L., Stephanopoulos, G., & Brugge, J. S. (2011). Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes & Development, 25(16), 1716–1733. https://doi.org/10.1101/gad.16771811
Harris, R. A., Bowker-Kinley, M. M., Huang, B., & Wu, P. (2002). Regulation of the activity of the pyruvate dehydrogenase complex. Advances in Enzyme Regulation, 42, 249–259. https://doi.org/10.1016/S0065-2571(01)00061-9
Hiller, S., DeKroon, R., Hamlett, E. D., Xu, L., Osorio, C., Robinette, J., Winnik, W., Simington, S., Maeda, N., Alzate, O., & Yi, X. (2016). Alpha-lipoic acid supplementation protects enzymes from damage by nitrosative and oxidative stress. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860(1), 36–45. https://doi.org/10.1016/j.bbagen.2015.09.001
Hiromasa, Y., Fujisawa, T., Aso, Y., & Roche, T. E. (2004). Organization of the Cores of the Mammalian Pyruvate Dehydrogenase Complex Formed by E2 and E2 Plus the E3-binding Protein and Their Capacities to Bind the E1 and E3 Components. Journal of Biological Chemistry, 279(8), 6921–6933. https://doi.org/10.1074/jbc.M308172200
Hiromasa, Y., Hu, L., & Roche, T. E. (2006). Ligand-induced Effects on Pyruvate Dehydrogenase Kinase Isoform 2. Journal of Biological Chemistry, 281(18), 12568–12579. https://doi.org/10.1074/jbc.M513514200
Hiromasa, Y., Yan, X., & Roche, T. E. (2008). Specific Ion Influences on Self-Association of Pyruvate Dehydrogenase Kinase Isoform 2 (PDHK2), Binding of PDHK2 to the L2 Lipoyl Domain, and Effects of the Lipoyl Group-Binding Site Inhibitor, Nov3r. Biochemistry, 47(8), 2312–2324. https://doi.org/10.1021/bi7014772
Hitosugi, T., Fan, J., Chung, T.-W., Lythgoe, K., Wang, X., Xie, J., Ge, Q., Gu, T.-L., Polakiewicz, R. D., Roesel, J. L., Chen, G. Z., Boggon, T. J., Lonial, S., Fu, H., Khuri, F. R., Kang, S., & Chen, J. (2011). Tyrosine Phosphorylation of Mitochondrial Pyruvate Dehydrogenase Kinase 1 Is Important for Cancer Metabolism. Molecular Cell, 44(6), 864–877. https://doi.org/10.1016/j.molcel.2011.10.015
Hol, W. G. J., Ævarsson, A., Seger, K., Turley, S., & Sokatch, J. R. (1999). Crystal structure of 2-oxoisovalerate dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nature Structural Biology, 6(8), 785–792. https://doi.org/10.1038/11563
Holness, M. J., & Sugden, M. C. (1989). Pyruvate dehydrogenase activities during the fed-to-starved transition and on re-feeding after acute or prolonged starvation. Biochemical Journal, 258(2), 529–533. https://doi.org/10.1042/bj2580529
Holness, M. J., & Sugden, M. C. (2003). Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochemical Society Transactions, 31(6), 1143–1151. https://doi.org/10.1042/bst0311143
Hong, S. M., Lee, Y.-K., Park, I., Kwon, S. M., Min, S., & Yoon, G. (2019). Lactic acidosis caused by repressed lactate dehydrogenase subunit B expression down-regulates mitochondrial oxidative phosphorylation via the pyruvate dehydrogenase (PDH)–PDH kinase axis. Journal of Biological Chemistry, 294(19), 7810–7820. https://doi.org/10.1074/jbc.RA118.006095
Huang, B., Wu, P., Bowker-Kinley, M. M., & Harris, R. A. (n.d.). Regulation of Pyruvate Dehydrogenase Kinase Expression by Peroxisome Proliferator–Activated Receptor- Ligands, Glucocorticoids, and Insulin.
Huang, D., Jing, G., & Zhu, S. (2023). Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants, 12(8), 1644. https://doi.org/10.3390/antiox12081644
Hue, L., & Taegtmeyer, H. (2009). The Randle cycle revisited: A new head for an old hat. American Journal of Physiology-Endocrinology and Metabolism, 297(3), E578–E591. https://doi.org/10.1152/ajpendo.00093.2009
Humphries, K. M., & Szweda, L. I. (1998). Selective Inactivation of α-Ketoglutarate Dehydrogenase and Pyruvate Dehydrogenase: Reaction of Lipoic Acid with 4-Hydroxy-2-nonenal. Biochemistry, 37(45), 15835–15841. https://doi.org/10.1021/bi981512h
Hurd, T. R., Collins, Y., Abakumova, I., Chouchani, E. T., Baranowski, B., Fearnley, I. M., Prime, T. A., Murphy, M. P., & James, A. M. (2012). Inactivation of Pyruvate Dehydrogenase Kinase 2 by Mitochondrial Reactive Oxygen Species. Journal of Biological Chemistry, 287(42), 35153–35160. https://doi.org/10.1074/jbc.M112.400002
Hwang, B., Jeoung, N. H., & Harris, R. A. (2009). Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochemical Journal, 423(2), 243–252. https://doi.org/10.1042/BJ20090390
Jane, E. P., Premkumar, D. R., Rajasundaram, D., Thambireddy, S., Reslink, M. C., Agnihotri, S., & Pollack, I. F. (2022). Reversing tozasertib resistance in glioma through inhibition of pyruvate dehydrogenase kinases. Molecular Oncology, 16(1), 219–249. https://doi.org/10.1002/1878-0261.13025
Jeong, J. Y., Jeoung, N. H., Park, K.-G., & Lee, I.-K. (2012). Transcriptional Regulation of Pyruvate Dehydrogenase Kinase. Diabetes & Metabolism Journal, 36(5), 328. https://doi.org/10.4093/dmj.2012.36.5.328
Jeoung, N. H. (2015). Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers. Diabetes & Metabolism Journal, 39(3), 188. https://doi.org/10.4093/dmj.2015.39.3.188
Jeoung, N. H., Harris, C. R., & Harris, R. A. (2014). Regulation of pyruvate metabolism in metabolic-related diseases. Reviews in Endocrine and Metabolic Disorders, 15(1), 99–110. https://doi.org/10.1007/s11154-013-9284-2
Jeoung, N. H., Rahimi, Y., Wu, P., Lee, W. N. P., & Harris, R. A. (2012). Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice. Biochemical Journal, 443(3), 829–839. https://doi.org/10.1042/BJ20112197
Jiang, W., Finniss, S., Cazacu, S., Xiang, C., Brodie, Z., Mikkelsen, T., Poisson, L., Shackelford, D. B., & Brodie, C. (2016). Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget, 7(35), 56456–56470. https://doi.org/10.18632/oncotarget.10919
Jordan, F. (2004). How Active Sites Communicate in Thiamine Enzymes. Science, 306(5697), 818–820. https://doi.org/10.1126/science.1105457
Jun, S., Mahesula, S., Mathews, T. P., Martin-Sandoval, M. S., Zhao, Z., Piskounova, E., & Agathocleous, M. (2021). The requirement for pyruvate dehydrogenase in leukemogenesis depends on cell lineage. Cell Metabolism, 33(9), 1777-1792.e8. https://doi.org/10.1016/j.cmet.2021.07.016
Kamarajugadda, S., Stemboroski, L., Cai, Q., Simpson, N. E., Nayak, S., Tan, M., & Lu, J. (2012). Glucose Oxidation Modulates Anoikis and Tumor Metastasis. Molecular and Cellular Biology, 32(10), 1893–1907. https://doi.org/10.1128/MCB.06248-11
Kaplon, J., Zheng, L., Meissl, K., Chaneton, B., Selivanov, V. A., Mackay, G., Van Der Burg, S. H., Verdegaal, E. M. E., Cascante, M., Shlomi, T., Gottlieb, E., & Peeper, D. S. (2013). A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature, 498(7452), 109–112. https://doi.org/10.1038/nature12154
Karpova, T., Danchuk, S., Kolobova, E., & Popov, K. M. (2003). Characterization of the isozymes of pyruvate dehydrogenase phosphatase: Implications for the regulation of pyruvate dehydrogenase activity. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1652(2), 126–135. https://doi.org/10.1016/j.bbapap.2003.08.010
Kato, M., Li, J., Chuang, J. L., & Chuang, D. T. (2007). Distinct Structural Mechanisms for Inhibition of Pyruvate Dehydrogenase Kinase Isoforms by AZD7545, Dichloroacetate, and Radicicol. Structure, 15(8), 992–1004. https://doi.org/10.1016/j.str.2007.07.001
Kato, M., Wynn, R. M., Chuang, J. L., Tso, S.-C., Machius, M., Li, J., & Chuang, D. T. (2008). Structural Basis for Inactivation of the Human Pyruvate Dehydrogenase Complex by Phosphorylation: Role of Disordered Phosphorylation Loops. Structure, 16(12), 1849–1859. https://doi.org/10.1016/j.str.2008.10.010
Kerbey, A. L., Randle, P. J., Cooper, R. H., Whitehouse, S., Pask, H. T., & Denton, R. M. (1976). Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: Role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochemical Journal, 154(2), 327–348. https://doi.org/10.1042/bj1540327
Kim, J., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3(3), 177–185. https://doi.org/10.1016/j.cmet.2006.02.002
Klose, K., Packeiser, E.-M., Müller, P., Granados-Soler, J. L., Schille, J. T., Goericke-Pesch, S., Kietzmann, M., Murua Escobar, H., & Nolte, I. (2021). Metformin and sodium dichloroacetate effects on proliferation, apoptosis, and metabolic activity tested alone and in combination in a canine prostate and a bladder cancer cell line. PLOS ONE, 16(9), e0257403. https://doi.org/10.1371/journal.pone.0257403
Kolobova, E., Tuganova, A., Boulatnikov, I., & Popov, K. M. (2001). Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochemical Journal, 358(1), 69–77. https://doi.org/10.1042/bj3580069
Korotchkina, L. G., & Patel, M. S. (1995). Mutagenesis Studies of the Phosphorylation Sites of Recombinant Human Pyruvate Dehydrogenase. Site-Specific Regulation. Journal of Biological Chemistry, 270(24), 14297–14304. https://doi.org/10.1074/jbc.270.24.14297
Korotchkina, L. G., & Patel, M. S. (2001a). Probing the Mechanism of Inactivation of Human Pyruvate Dehydrogenase by Phosphorylation of Three Sites. Journal of Biological Chemistry, 276(8), 5731–5738. https://doi.org/10.1074/jbc.M007558200
Korotchkina, L. G., & Patel, M. S. (2001b). Site Specificity of Four Pyruvate Dehydrogenase Kinase Isoenzymes toward the Three Phosphorylation Sites of Human Pyruvate Dehydrogenase. Journal of Biological Chemistry, 276(40), 37223–37229. https://doi.org/10.1074/jbc.M103069200
Korotchkina, L. G., Sidhu, S., & Patel, M. S. (2004). R-Lipoic Acid Inhibits Mammalian Pyruvate Dehydrogenase Kinase. Free Radical Research, 38(10), 1083–1092. https://doi.org/10.1080/10715760400004168
Koukourakis, M. I., Giatromanolaki, A., Sivridis, E., Gatter, K. C., & Harris, A. L. (2005). Pyruvate Dehydrogenase and Pyruvate Dehydrogenase Kinase Expression in Non Small Cell Lung Cancer and Tumor-Associated Stroma. Neoplasia, 7(1), 1–6. https://doi.org/10.1593/neo.04373
Kuntz, M. J., & Harris, R. A. (2018). Pyruvate Dehydrogenase Kinase. In S. Choi (Ed.), Encyclopedia of Signaling Molecules (pp. 1–9). Springer New York. https://doi.org/10.1007/978-1-4614-6438-9_101636-1
Kyrilis, F. L., Semchonok, D. A., Skalidis, I., Tüting, C., Hamdi, F., O’Reilly, F. J., Rappsilber, J., & Kastritis, P. L. (2021). Integrative structure of a 10-megadalton eukaryotic pyruvate dehydrogenase complex from native cell extracts. Cell Reports, 34(6), 108727. https://doi.org/10.1016/j.celrep.2021.108727
Lazzarino, G., Amorini, A. M., Signoretti, S., Musumeci, G., Lazzarino, G., Caruso, G., Pastore, F. S., Di Pietro, V., Tavazzi, B., & Belli, A. (2019). Pyruvate Dehydrogenase and Tricarboxylic Acid Cycle Enzymes Are Sensitive Targets of Traumatic Brain Injury Induced Metabolic Derangement. International Journal of Molecular Sciences, 20(22), 5774. https://doi.org/10.3390/ijms20225774
Lee, J., Oh, S., Bhattacharya, S., Zhang, Y., Florens, L., Washburn, M. P., & Workman, J. L. (2020). The plasticity of the pyruvate dehydrogenase complex confers a labile structure that is associated with its catalytic activity. PLOS ONE, 15(12), e0243489. https://doi.org/10.1371/journal.pone.0243489
Lengyel, J. S., Stott, K. M., Wu, X., Brooks, B. R., Balbo, A., Schuck, P., Perham, R. N., Subramaniam, S., & Milne, J. L. S. (2008). Extended Polypeptide Linkers Establish the Spatial Architecture of a Pyruvate Dehydrogenase Multienzyme Complex. Structure, 16(1), 93–103. https://doi.org/10.1016/j.str.2007.10.017
Li, J., Machius, M., Chuang, J. L., Wynn, R. M., & Chuang, D. T. (2007). The Two Active Sites in Human Branched-chain α-Keto Acid Dehydrogenase Operate Independently without an Obligatory Alternating-site Mechanism. Journal of Biological Chemistry, 282(16), 11904–11913. https://doi.org/10.1074/jbc.M610843200
Li, X., Jiang, Y., Meisenhelder, J., Yang, W., Hawke, D. H., Zheng, Y., Xia, Y., Aldape, K., He, J., Hunter, T., Wang, L., & Lu, Z. (2016). Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Molecular Cell, 61(5), 705–719. https://doi.org/10.1016/j.molcel.2016.02.009
Li, Y., Shen, J., Cheng, C., Gao, H., Zhao, J., & Chen, L. (2020). Overexpression of pyruvate dehydrogenase phosphatase 1 promotes the progression of pancreatic adenocarcinoma by regulating energy-related AMPK/mTOR signaling. Cell & Bioscience, 10(1), 95. https://doi.org/10.1186/s13578-020-00457-5
Liu, S., Xia, X., Zhen, J., Li, Z., & Zhou, Z. H. (2022). Structures and comparison of endogenous 2-oxoglutarate and pyruvate dehydrogenase complexes from bovine kidney. Cell Discovery, 8(1), 126. https://doi.org/10.1038/s41421-022-00487-y
Long, D. L., McCall, C. E., & Poole, L. B. (2023). Glutathionylation of Pyruvate Dehydrogenase Complex E2 and Inflammatory Cytokine Production During Acute Inflammation Are Magnified By Mitochondrial Oxidative Stress. Immunology. https://doi.org/10.1101/2023.01.26.525791
Lu, C.-W., Lin, S.-C., Chen, K.-F., Lai, Y.-Y., & Tsai, S.-J. (2008). Induction of Pyruvate Dehydrogenase Kinase-3 by Hypoxia-inducible Factor-1 Promotes Metabolic Switch and Drug Resistance. Journal of Biological Chemistry, 283(42), 28106–28114. https://doi.org/10.1074/jbc.M803508200
Lu, C.-W., Lin, S.-C., Chien, C.-W., Lin, S.-C., Lee, C.-T., Lin, B.-W., Lee, J.-C., & Tsai, S.-J. (2011). Overexpression of Pyruvate Dehydrogenase Kinase 3 Increases Drug Resistance and Early Recurrence in Colon Cancer. The American Journal of Pathology, 179(3), 1405–1414. https://doi.org/10.1016/j.ajpath.2011.05.050
Lu, H., Lu, Y., Xie, Y., Qiu, S., Li, X., & Fan, Z. (2019). Rational combination with PDK1 inhibition overcomes cetuximab resistance in head and neck squamous cell carcinoma. JCI Insight, 4(19), e131106. https://doi.org/10.1172/jci.insight.131106
-
Mailloux, R. J., Gardiner, D., & O’Brien, M. (2016). 2-Oxoglutarate dehydrogenase is a more significant source of O2·−/H2O2 than pyruvate dehydrogenase in cardiac and liver tissue. Free Radical Biology and Medicine, 97, 501–512. https://doi.org/10.1016/j.freeradbiomed.2016.06.014
Maj, M. C., Cameron, J. M., & Robinson, B. H. (2006). Pyruvate dehydrogenase phosphatase deficiency: Orphan disease or an under-diagnosed condition? Molecular and Cellular Endocrinology, 249(1–2), 1–9. https://doi.org/10.1016/j.mce.2006.02.003
Masini, T., Birkaya, B., Van Dijk, S., Mondal, M., Hekelaar, J., Jäger, M., Terwisscha Van Scheltinga, A. C., Patel, M. S., Hirsch, A. K. H., & Moman, E. (2016). Furoates and thenoates inhibit pyruvate dehydrogenase kinase 2 allosterically by binding to its pyruvate regulatory site. Journal of Enzyme Inhibition and Medicinal Chemistry, 31(sup4), 170–175. https://doi.org/10.1080/14756366.2016.1201812
Mathias, R. A., Greco, T. M., Oberstein, A., Budayeva, H. G., Chakrabarti, R., Rowland, E. A., Kang, Y., Shenk, T., & Cristea, I. M. (2014). Sirtuin 4 Is a Lipoamidase Regulating Pyruvate Dehydrogenase Complex Activity. Cell, 159(7), 1615–1625. https://doi.org/10.1016/j.cell.2014.11.046
Mayr, J. A., Feichtinger, R. G., Tort, F., Ribes, A., & Sperl, W. (2014). Lipoic acid biosynthesis defects. Journal of Inherited Metabolic Disease, 37(4), 553–563. https://doi.org/10.1007/s10545-014-9705-8
McFate, T., Mohyeldin, A., Lu, H., Thakar, J., Henriques, J., Halim, N. D., Wu, H., Schell, M. J., Tsang, T. M., Teahan, O., Zhou, S., Califano, J. A., Jeoung, N. H., Harris, R. A., & Verma, A. (2008). Pyruvate Dehydrogenase Complex Activity Controls Metabolic and Malignant Phenotype in Cancer Cells. Journal of Biological Chemistry, 283(33), 22700–22708. https://doi.org/10.1074/jbc.M801765200
McKelvey, K. J., Wilson, E. B., Short, S., Melcher, A. A., Biggs, M., Diakos, C. I., & Howell, V. M. (2021). Glycolysis and Fatty Acid Oxidation Inhibition Improves Survival in Glioblastoma. Frontiers in Oncology, 11, 633210. https://doi.org/10.3389/fonc.2021.633210
McLean, P., Kunjara, S., Greenbaum, A. L., Gumaa, K., López-Prados, J., Martin-Lomas, M., & Rademacher, T. W. (2008). Reciprocal Control of Pyruvate Dehydrogenase Kinase and Phosphatase by Inositol Phosphoglycans: Dynamic State Set by “Push-Pull” System. Journal of Biological Chemistry, 283(48), 33428–33436. https://doi.org/10.1074/jbc.M801781200
Mehr, A. (2023). Structural interrogation of enzyme mechanism and dynamics [Georg-August-University Göttingen]. https://doi.org/10.53846/goediss-9875
Milne, J. L. S. (2002). Molecular architecture and mechanism of an icosahedral pyruvate dehydrogenase complex: A multifunctional catalytic machine. The EMBO Journal, 21(21), 5587–5598. https://doi.org/10.1093/emboj/cdf574
Milne, J. L. S., Wu, X., Borgnia, M. J., Lengyel, J. S., Brooks, B. R., Shi, D., Perham, R. N., & Subramaniam, S. (2006). Molecular Structure of a 9-MDa Icosahedral Pyruvate Dehydrogenase Subcomplex Containing the E2 and E3 Enzymes Using Cryoelectron Microscopy. Journal of Biological Chemistry, 281(7), 4364–4370. https://doi.org/10.1074/jbc.M504363200
Moore, J. D., Staniszewska, A., Shaw, T., D’Alessandro, J., Davis, B., Surgenor, A., Baker, L., Matassova, N., Murray, J., Brough, P., Wood, M., & Mahon, P. C. (n.d.). VER-246608, a novel pan-isoform ATP competitive inhibitor of pyruvate dehydrogenase kinase, disrupts Warburg metabolism and induces context-dependent cytostasis in cancer cells.
Motojima, K., & Seto, K. (2003). Fibrates and Statins Rapidly and Synergistically Induce Pyruvate Dehydrogenase Kinase 4 mRNA in the Liver and Muscles of Mice. Biological and Pharmaceutical Bulletin, 26(7), 954–958. https://doi.org/10.1248/bpb.26.954
Nemeria, N., Arjunan, P., Brunskill, A., Sheibani, F., Wei, W., Yan, Zhang, S., Jordan, F., & Furey, W. (2002). Histidine 407, a Phantom Residue in the E1 Subunit of the Escherichia coli Pyruvate Dehydrogenase Complex, Activates Reductive Acetylation of Lipoamide on the E2 Subunit. An Explanation for Conservation of Active Sites between the E1 Subunit and Transketolase. Biochemistry, 41(52), 15459–15467. https://doi.org/10.1021/bi0205909
Nemeria, N. S., Ambrus, A., Patel, H., Gerfen, G., Adam-Vizi, V., Tretter, L., Zhou, J., Wang, J., & Jordan, F. (2014). Human 2-Oxoglutarate Dehydrogenase Complex E1 Component Forms a Thiamin-derived Radical by Aerobic Oxidation of the Enamine Intermediate. Journal of Biological Chemistry, 289(43), 29859–29873. https://doi.org/10.1074/jbc.M114.591073
Nemeria, N. S., Chakraborty, S., Balakrishnan, A., & Jordan, F. (2009). Reaction mechanisms of thiamin diphosphate enzymes: Defining states of ionization and tautomerization of the cofactor at individual steps. The FEBS Journal, 276(9), 2432–3446. https://doi.org/10.1111/j.1742-4658.2009.06964.x
Norton, L., & DeFronzo, R. (2014). Skeletal Muscle Glucose Metabolism and Insulin Resistance. In Pathobiology of Human Disease (pp. 477–487). Elsevier. https://doi.org/10.1016/B978-0-12-386456-7.02003-7
Olson, M. S., Hampson, R. K., & Craig, F. (1986). Regulation of the hepatic glycine-cleavage system. Biochemical Society Transactions, 14(6), 1004–1005. https://doi.org/10.1042/bst0141004
O’Reilly, F. J., Graziadei, A., Forbrig, C., Bremenkamp, R., Charles, K., Lenz, S., Elfmann, C., Fischer, L., Stülke, J., & Rappsilber, J. (2023). Protein complexes in cells by AI‐assisted structural proteomics. Molecular Systems Biology, 19(4), e11544. https://doi.org/10.15252/msb.202311544
Orfali, K. A., Fryer, L. G. D., Holness, M. J., & Sugden, M. C. (1993). Long‐term regulation of pyruvate dehydrogenase kinase by high‐fat feeding: Experiments in vivo and in cultured cardiomyocytes. FEBS Letters, 336(3), 501–505. https://doi.org/10.1016/0014-5793(93)80864-Q
Patel, K. P., O’Brien, T. W., Subramony, S. H., Shuster, J., & Stacpoole, P. W. (2012). The spectrum of pyruvate dehydrogenase complex deficiency: Clinical, biochemical and genetic features in 371 patients. Molecular Genetics and Metabolism, 105(1), 34–43. https://doi.org/10.1016/j.ymgme.2011.09.032
Patel, M. S., & Korotchkina, L. G. (n.d.). Regulation of the pyruvate dehydrogenase complex.
Patel, M. S., & Korotchkina, L. G. (2001). Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: Complexity of multiple phosphorylation sites and kinases. Experimental & Molecular Medicine, 33(4), 191–197. https://doi.org/10.1038/emm.2001.32
Patel, M. S., Nemeria, N. S., Furey, W., & Jordan, F. (2014). The Pyruvate Dehydrogenase Complexes: Structure-based Function and Regulation. Journal of Biological Chemistry, 289(24), 16615–16623. https://doi.org/10.1074/jbc.R114.563148
Patel, M. S., & Roche, T. E. (1990). Molecular biology and biochemistry of pyruvate dehydrogenase complexes. The FASEB Journal, 4(14), 3224–3233. https://doi.org/10.1096/fasebj.4.14.2227213
Pavlu-Pereira, H., Lousa, D., Tomé, C. S., Florindo, C., Silva, M. J., De Almeida, I. T., Leandro, P., Rivera, I., & Vicente, J. B. (2021). Structural and functional impact of clinically relevant E1α variants causing pyruvate dehydrogenase complex deficiency. Biochimie, 183, 78–88. https://doi.org/10.1016/j.biochi.2021.02.007
Pawelczyk, T., & Olson, M. S. (1995). Changes in the structure of pyruvate dehydrogenase complex induced by mono- and divalent ions. The International Journal of Biochemistry & Cell Biology, 27(5), 513–521. https://doi.org/10.1016/1357-2725(95)00006-B
Paxton, R., Scislowski, P. W., Davis, E. J., & Harris, R. A. (1986). Role of branched-chain 2-oxo acid dehydrogenase and pyruvate dehydrogenase in 2-oxobutyrate metabolism. Biochemical Journal, 234(2), 295–303. https://doi.org/10.1042/bj2340295
Pei, X. Y., Titman, C. M., Frank, R. A. W., Leeper, F. J., & Luisi, B. F. (2008). Snapshots of Catalysis in the E1 Subunit of the Pyruvate Dehydrogenase Multienzyme Complex. Structure, 16(12), 1860–1872. https://doi.org/10.1016/j.str.2008.10.009
Perham, R. N. (2000). Swinging Arms and Swinging Domains in Multifunctional Enzymes: Catalytic Machines for Multistep Reactions. Annual Review of Biochemistry, 69(1), 961–1004. https://doi.org/10.1146/annurev.biochem.69.1.961
Peters, S. J., & LeBlanc, P. J. (2004). Metabolic aspects of low carbohydrate diets and exercise. Nutrition & Metabolism, 1(1), 7. https://doi.org/10.1186/1743-7075-1-7
Pin, F., Novinger, L. J., Huot, J. R., Harris, R. A., Couch, M. E., O’Connell, T. M., & Bonetto, A. (2019). PDK4 drives metabolic alterations and muscle atrophy in cancer cachexia. The FASEB Journal, 33(6), 7778–7790. https://doi.org/10.1096/fj.201802799R
Prabhu, A., Sarcar, B., Miller, C. R., Kim, S.-H., Nakano, I., Forsyth, P., & Chinnaiyan, P. (2015). Ras-mediated modulation of pyruvate dehydrogenase activity regulates mitochondrial reserve capacity and contributes to glioblastoma tumorigenesis. Neuro-Oncology, 17(9), 1220–1230. https://doi.org/10.1093/neuonc/nou369
Prajapati, S., Haselbach, D., Wittig, S., Patel, M. S., Chari, A., Schmidt, C., Stark, H., & Tittmann, K. (2019). Structural and Functional Analyses of the Human PDH Complex Suggest a “Division-of-Labor” Mechanism by Local E1 and E3 Clusters. Structure, 27(7), 1124-1136.e4. https://doi.org/10.1016/j.str.2019.04.009
Prajapati, S., Rabe Von Pappenheim, F., & Tittmann, K. (2022). Frontiers in the enzymology of thiamin diphosphate-dependent enzymes. Current Opinion in Structural Biology, 76, 102441. https://doi.org/10.1016/j.sbi.2022.102441
Priestman, D. A., Orfali, K. A., & Sugden, M. C. (1996). Pyruvate inhibition of pyruvate dehydrogenase kinase: Effects of progressive starvation and hyperthyroidism in vivo, and of dibutyryl cyclic AMP and fatty acids in cultured cardiac myocytes. FEBS Letters, 393(2–3), 174–178. https://doi.org/10.1016/0014-5793(96)00877-0
Prochownik, E. V., & Wang, H. (2021). The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells. Cells, 10(4), 762. https://doi.org/10.3390/cells10040762
Quinlan, C. L., Goncalves, R. L. S., Hey-Mogensen, M., Yadava, N., Bunik, V. I., & Brand, M. D. (2014). The 2-Oxoacid Dehydrogenase Complexes in Mitochondria Can Produce Superoxide/Hydrogen Peroxide at Much Higher Rates Than Complex I. Journal of Biological Chemistry, 289(12), 8312–8325. https://doi.org/10.1074/jbc.M113.545301
Roche, T. E., Baker, J. C., Yan, X., Hiromasa, Y., Gong, X., Peng, T., Dong, J., Turkan, A., & Kasten, S. A. (2001). Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. In Progress in Nucleic Acid Research and Molecular Biology (Vol. 70, pp. 33–75). Elsevier. https://doi.org/10.1016/S0079-6603(01)70013-X
Roche, T. E., & Hiromasa, Y. (2007). Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cellular and Molecular Life Sciences, 64(7–8), 830–849. https://doi.org/10.1007/s00018-007-6380-z
Roche, T. E., Hiromasa, Y., Turkan, A., Gong, X., Peng, T., Yan, X., Kasten, S. A., Bao, H., & Dong, J. (2003). Essential roles of lipoyl domains in the activated function and control of pyruvate dehydrogenase kinases and phosphatase isoform 1. European Journal of Biochemistry, 270(6), 1050–1056. https://doi.org/10.1046/j.1432-1033.2003.03468.x
Roche, T. E., & Reed, L. J. (1974). Monovalent cation requirement for ADP inhibition of pyruvate dehydrogenase kinase. Biochemical and Biophysical Research Communications, 59(4), 1341–1348. https://doi.org/10.1016/0006-291X(74)90461-6
Roosterman, D., Meyerhof, W., & Cottrell, G. S. (2018). Proton Transport Chains in Glucose Metabolism: Mind the Proton. Frontiers in Neuroscience, 12, 404. https://doi.org/10.3389/fnins.2018.00404
Sale, G. J., & Randle, P. J. (1982). Occupancy of phosphorylation sites in pyruvate dehydrogenase phosphate complex in rat heart in vivo. Relation to proportion of inactive complex and rate of re-activation by phosphatase. Biochemical Journal, 206(2), 221–229. https://doi.org/10.1042/bj2060221
Sattler, U. G. A., & Mueller-Klieser, W. (2009). The anti-oxidant capacity of tumour glycolysis. International Journal of Radiation Biology, 85(11), 963–971. https://doi.org/10.3109/09553000903258889
Saunier, E., Benelli, C., & Bortoli, S. (2016). The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. International Journal of Cancer, 138(4), 809–817. https://doi.org/10.1002/ijc.29564
Schell, J. C., Olson, K. A., Jiang, L., Hawkins, A. J., Van Vranken, J. G., Xie, J., Egnatchik, R. A., Earl, E. G., DeBerardinis, R. J., & Rutter, J. (2014). A Role for the Mitochondrial Pyruvate Carrier as a Repressor of the Warburg Effect and Colon Cancer Cell Growth. Molecular Cell, 56(3), 400–413. https://doi.org/10.1016/j.molcel.2014.09.026
Schell, J. C., Wisidagama, D. R., Bensard, C., Zhao, H., Wei, P., Tanner, J., Flores, A., Mohlman, J., Sorensen, L. K., Earl, C. S., Olson, K. A., Miao, R., Waller, T. C., Delker, D., Kanth, P., Jiang, L., DeBerardinis, R. J., Bronner, M. P., Li, D. Y., … Rutter, J. (2017). Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nature Cell Biology, 19(9), 1027–1036. https://doi.org/10.1038/ncb3593
Schoonjans, C. A., Joudiou, N., Brusa, D., Corbet, C., Feron, O., & Gallez, B. (2020). Acidosis-induced metabolic reprogramming in tumor cells enhances the anti-proliferative activity of the PDK inhibitor dichloroacetate. Cancer Letters, 470, 18–28. https://doi.org/10.1016/j.canlet.2019.12.003
Schröder-Tittmann, K., Meyer, D., Arens, J., Wechsler, C., Tietzel, M., Golbik, R., & Tittmann, K. (2013). Alternating Sites Reactivity Is a Common Feature of Thiamin Diphosphate-Dependent Enzymes As Evidenced by Isothermal Titration Calorimetry Studies of Substrate Binding. Biochemistry, 52(15), 2505–2507. https://doi.org/10.1021/bi301591e
Schulze, A., & Downward, J. (2011). Flicking the Warburg Switch—Tyrosine Phosphorylation of Pyruvate Dehydrogenase Kinase Regulates Mitochondrial Activity in Cancer Cells. Molecular Cell, 44(6), 846–848. https://doi.org/10.1016/j.molcel.2011.12.004
Schwartz. (2010). A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: Preliminary results. Oncology Reports, 23(5). https://doi.org/10.3892/or_00000778
Schwer, B., Eckersdorff, M., Li, Y., Silva, J. C., Fermin, D., Kurtev, M. V., Giallourakis, C., Comb, M. J., Alt, F. W., & Lombard, D. B. (2009). Calorie restriction alters mitochondrial protein acetylation. Aging Cell, 8(5), 604–606. https://doi.org/10.1111/j.1474-9726.2009.00503.x
Sebastian, C., Ferrer, C., Serra, M., Choi, J.-E., Ducano, N., Mira, A., Shah, M. S., Stopka, S. A., Perciaccante, A. J., Isella, C., Moya-Rull, D., Vara-Messler, M., Giordano, S., Maldi, E., Desai, N., Capen, D. E., Medico, E., Cetinbas, M., Sadreyev, R. I., … Mostoslavsky, R. (2022). A non-dividing cell population with high pyruvate dehydrogenase kinase activity regulates metabolic heterogeneity and tumorigenesis in the intestine. Nature Communications, 13(1), 1503. https://doi.org/10.1038/s41467-022-29085-y
Seifert, F., Ciszak, E., Korotchkina, L., Golbik, R., Spinka, M., Dominiak, P., Sidhu, S., Brauer, J., Patel, M. S., & Tittmann, K. (2007). Phosphorylation of Serine 264 Impedes Active Site Accessibility in the E1 Component of the Human Pyruvate Dehydrogenase Multienzyme Complex. Biochemistry, 46(21), 6277–6287. https://doi.org/10.1021/bi700083z
Seifert, F., Golbik, R., Brauer, J., Lilie, H., Schröder-Tittmann, K., Hinze, E., Korotchkina, L. G., Patel, M. S., & Tittmann, K. (2006). Direct Kinetic Evidence for Half-Of-The-Sites Reactivity in the E1 Component of the Human Pyruvate Dehydrogenase Multienzyme Complex through Alternating Sites Cofactor Activation. Biochemistry, 45(42), 12775–12785. https://doi.org/10.1021/bi061582l
Seim, G. L., John, S. V., Arp, N. L., Fang, Z., Pagliarini, D. J., & Fan, J. (2023). Nitric oxide-driven modifications of lipoic arm inhibit α-ketoacid dehydrogenases. Nature Chemical Biology, 19(3), 265–274. https://doi.org/10.1038/s41589-022-01153-w
Sgrignani, J., Chen, J., Alimonti, A., & Cavalli, A. (2018). How phosphorylation influences E1 subunit pyruvate dehydrogenase: A computational study. Scientific Reports, 8(1), 14683. https://doi.org/10.1038/s41598-018-33048-z
Shan, C., Kang, H.-B., Elf, S., Xie, J., Gu, T.-L., Aguiar, M., Lonning, S., Hitosugi, T., Chung, T.-W., Arellano, M., Khoury, H. J., Shin, D. M., Khuri, F. R., Boggon, T. J., & Fan, J. (2014). Tyr-94 Phosphorylation Inhibits Pyruvate Dehydrogenase Phosphatase 1 and Promotes Tumor Growth. Journal of Biological Chemistry, 289(31), 21413–21422. https://doi.org/10.1074/jbc.M114.581124
Skalidis, I., Tüting, C., & Kastritis, P. L. (2020). Unstructured regions of large enzymatic complexes control the availability of metabolites with signaling functions. Cell Communication and Signaling, 18(1), 136. https://doi.org/10.1186/s12964-020-00631-9
Škerlová, J., Berndtsson, J., Nolte, H., Ott, M., & Stenmark, P. (2021). Structure of the native pyruvate dehydrogenase complex reveals the mechanism of substrate insertion. Nature Communications, 12(1), 5277. https://doi.org/10.1038/s41467-021-25570-y
Smith, E. R., & Hewitson, T. D. (2020). TGF-β1 is a regulator of the pyruvate dehydrogenase complex in fibroblasts. Scientific Reports, 10(1), 17914. https://doi.org/10.1038/s41598-020-74919-8
Smolle, M., Prior, A. E., Brown, A. E., Cooper, A., Byron, O., & Lindsay, J. G. (2006). A New Level of Architectural Complexity in the Human Pyruvate Dehydrogenase Complex. Journal of Biological Chemistry, 281(28), 19772–19780. https://doi.org/10.1074/jbc.M601140200
Solmonson, A., & DeBerardinis, R. J. (2018). Lipoic acid metabolism and mitochondrial redox regulation. Journal of Biological Chemistry, 293(20), 7522–7530. https://doi.org/10.1074/jbc.TM117.000259
Song, J., & Jordan, F. (2012). Interchain Acetyl Transfer in the E2 Component of Bacterial Pyruvate Dehydrogenase Suggests a Model with Different Roles for Each Chain in a Trimer of the Homooligomeric Component. Biochemistry, 51(13), 2795–2803. https://doi.org/10.1021/bi201614n
Srivastava, N., Kollipara, R. K., Singh, D. K., Sudderth, J., Hu, Z., Nguyen, H., Wang, S., Humphries, C. G., Carstens, R., Huffman, K. E., DeBerardinis, R. J., & Kittler, R. (2014). Inhibition of Cancer Cell Proliferation by PPARγ Is Mediated by a Metabolic Switch that Increases Reactive Oxygen Species Levels. Cell Metabolism, 20(4), 650–661. https://doi.org/10.1016/j.cmet.2014.08.003
Stacpoole, P. W. (2011). The Dichloroacetate Dilemma: Environmental Hazard versus Therapeutic Goldmine—Both or Neither? Environmental Health Perspectives, 119(2), 155–158. https://doi.org/10.1289/ehp.1002554
Stacpoole, P. W. (2012). The pyruvate dehydrogenase complex as a therapeutic target for age‐related diseases. Aging Cell, 11(3), 371–377. https://doi.org/10.1111/j.1474-9726.2012.00805.x
Stacpoole, P. W. (2017). Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. JNCI: Journal of the National Cancer Institute, 109(11). https://doi.org/10.1093/jnci/djx071
Stacpoole, P. W., & McCall, C. E. (2023). The pyruvate dehydrogenase complex: Life’s essential, vulnerable and druggable energy homeostat. Mitochondrion, 70, 59–102. https://doi.org/10.1016/j.mito.2023.02.007
Strowitzki, M. J., Radhakrishnan, P., Pavicevic, S., Scheer, J., Kimmer, G., Ritter, A. S., Tuffs, C., Volz, C., Vondran, F., Harnoss, J. M., Klose, J., Schmidt, T., & Schneider, M. (2019). High hepatic expression of PDK4 improves survival upon multimodal treatment of colorectal liver metastases. British Journal of Cancer, 120(7), 675–688. https://doi.org/10.1038/s41416-019-0406-9
Sugden, C. M., Fryer, G. D. L., Orfali, A. K., Priestman, A. D., Donald, E., & Holness, J. M. (1998). Studies of the long-term regulation of hepatic pyruvate dehydrogenase kinase. Biochemical Journal, 329(1), 89–94. https://doi.org/10.1042/bj3290089
Sugden, M. C. (2008). PDC deletion: The way to a man’s heart disease. American Journal of Physiology-Heart and Circulatory Physiology, 295(3), H917–H919. https://doi.org/10.1152/ajpheart.00663.2008
Sugden, M. C., & Holness, M. J. (1989). Effects of re-feeding after prolonged starvation on pyruvate dehydrogenase activities in heart, diaphragm and selected skeletal muscles of the rat. Biochemical Journal, 262(2), 669–672. https://doi.org/10.1042/bj2620669
Sugden, M. C., & Holness, M. J. (2003). Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. American Journal of Physiology-Endocrinology and Metabolism, 284(5), E855–E862. https://doi.org/10.1152/ajpendo.00526.2002
Sugden, M. C., & Holness, M. J. (2006). Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Archives of Physiology and Biochemistry, 112(3), 139–149. https://doi.org/10.1080/13813450600935263
Sugden, M. C., Orfali, K. A., & Holness, M. J. (1995). The Pyruvate Dehydrogenase Complex: Nutrient Control and the Pathogenesis of Insulin Resistance. The Journal of Nutrition, 125, 1746S-1752S. https://doi.org/10.1093/jn/125.suppl_6.1746S
Sugden, P. H., Hutson, N. J., Kerbey, A. L., & Randle, P. J. (1978). Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochemical Journal, 169(2), 433–435. https://doi.org/10.1042/bj1690433
Sugden, P. H., & Randle, P. J. (1978). Regulation of pig heart pyruvate dehydrogenase by phosphorylation. Studies on the subunit and phosphorylation stoicheiometries. Biochemical Journal, 173(2), 659–668. https://doi.org/10.1042/bj1730659
Sugden, P. H., & Simister, N. E. (1980). Role of multisite phosphorylation in the regulation of ox kidney pyruvate dehydrogenase complex. FEBS Letters, 111(2), 299–302. https://doi.org/10.1016/0014-5793(80)80814-3
Sutendra, G., Kinnaird, A., Dromparis, P., Paulin, R., Stenson, T. H., Haromy, A., Hashimoto, K., Zhang, N., Flaim, E., & Michelakis, E. D. (2014). A Nuclear Pyruvate Dehydrogenase Complex Is Important for the Generation of Acetyl-CoA and Histone Acetylation. Cell, 158(1), 84–97. https://doi.org/10.1016/j.cell.2014.04.046
Sutendra, G., & Michelakis, E. D. (2013). Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Frontiers in Oncology, 3. https://doi.org/10.3389/fonc.2013.00038
Takakusagi, Y., Matsumoto, S., Saito, K., Matsuo, M., Kishimoto, S., Wojtkowiak, J. W., DeGraff, W., Kesarwala, A. H., Choudhuri, R., Devasahayam, N., Subramanian, S., Munasinghe, J. P., Gillies, R. J., Mitchell, J. B., Hart, C. P., & Krishna, M. C. (2014). Pyruvate Induces Transient Tumor Hypoxia by Enhancing Mitochondrial Oxygen Consumption and Potentiates the Anti-Tumor Effect of a Hypoxia-Activated Prodrug TH-302. PLoS ONE, 9(9), e107995. https://doi.org/10.1371/journal.pone.0107995
Tovar‐Méndez, A., Miernyk, J. A., & Randall, D. D. (2003). Regulation of pyruvate dehydrogenase complex activity in plant cells. European Journal of Biochemistry, 270(6), 1043–1049. https://doi.org/10.1046/j.1432-1033.2003.03469.x
Tso, S.-C., Qi, X., Gui, W.-J., Wu, C.-Y., Chuang, J. L., Wernstedt-Asterholm, I., Morlock, L. K., Owens, K. R., Scherer, P. E., Williams, N. S., Tambar, U. K., Wynn, R. M., & Chuang, D. T. (2014). Structure-guided Development of Specific Pyruvate Dehydrogenase Kinase Inhibitors Targeting the ATP-binding Pocket. Journal of Biological Chemistry, 289(7), 4432–4443. https://doi.org/10.1074/jbc.M113.533885
Tsvetkov, P., Coy, S., Petrova, B., Dreishpoon, M., Verma, A., Abdusamad, M., Rossen, J., Joesch-Cohen, L., Humeidi, R., Spangler, R. D., Eaton, J. K., Frenkel, E., Kocak, M., Corsello, S. M., Lutsenko, S., Kanarek, N., Santagata, S., & Golub, T. R. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science, 375(6586), 1254–1261. https://doi.org/10.1126/science.abf0529
Turvey, E. A., Heigenhauser, G. J. F., Parolin, M., & Peters, S. J. (2005). Elevated n-3 fatty acids in a high-fat diet attenuate the increase in PDH kinase activity but not PDH activity in human skeletal muscle. J Appl Physiol, 98.
Tüting, C., Kyrilis, F. L., Müller, J., Sorokina, M., Skalidis, I., Hamdi, F., Sadian, Y., & Kastritis, P. L. (2021). Cryo-EM snapshots of a native lysate provide structural insights into a metabolon-embedded transacetylase reaction. Nature Communications, 12(1), 6933. https://doi.org/10.1038/s41467-021-27287-4
Wada, H., Shintani, D., & Ohlrogge, J. (1997). Why do mitochondria synthesize fatty acids? Evidence for involvement in lipoic acid production. Proceedings of the National Academy of Sciences, 94(4), 1591–1596. https://doi.org/10.1073/pnas.94.4.1591
Wang, C., Ma, C., Xu, Y., Chang, S., Wu, H., Yan, C., Chen, J., Wu, Y., An, S., Xu, J., Han, Q., Jiang, Y., Jiang, Z., Chu, X., Gao, H., Zhang, X., & Chang, Y. (2025). Dynamics of the mammalian pyruvate dehydrogenase complex revealed by in-situ structural analysis. Nature Communications, 16(1), 917. https://doi.org/10.1038/s41467-025-56171-8
Wang, X., Shen, X., Yan, Y., & Li, H. (2021). Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Bioscience Reports, 41(4), BSR20204402. https://doi.org/10.1042/BSR20204402
Whitley, M. J., Arjunan, P., Nemeria, N. S., Korotchkina, L. G., Park, Y.-H., Patel, M. S., Jordan, F., & Furey, W. (2018). Pyruvate dehydrogenase complex deficiency is linked to regulatory loop disorder in the αV138M variant of human pyruvate dehydrogenase. Journal of Biological Chemistry, 293(34), 13204–13213. https://doi.org/10.1074/jbc.RA118.003996
Woolbright, B. L., & Harris, R. A. (2021). PDK2: An Underappreciated Regulator of Liver Metabolism. Livers, 1(2), 82–97. https://doi.org/10.3390/livers1020008
Woolbright, B. L., Rajendran, G., Harris, R. A., & Taylor, J. A. (2019). Metabolic Flexibility in Cancer: Targeting the Pyruvate Dehydrogenase Kinase:Pyruvate Dehydrogenase Axis. Molecular Cancer Therapeutics, 18(10), 1673–1681. https://doi.org/10.1158/1535-7163.MCT-19-0079
Wu, P., Blair, P. V., Sato, J., Jaskiewicz, J., Popov, K. M., & Harris, R. A. (2000). Starvation Increases the Amount of Pyruvate Dehydrogenase Kinase in Several Mammalian Tissues. Archives of Biochemistry and Biophysics, 381(1), 1–7. https://doi.org/10.1006/abbi.2000.1946
Wynn, R. M., Kato, M., Chuang, J. L., Tso, S.-C., Li, J., & Chuang, D. T. (2008). Pyruvate Dehydrogenase Kinase-4 Structures Reveal a Metastable Open Conformation Fostering Robust Core-free Basal Activity. Journal of Biological Chemistry, 283(37), 25305–25315. https://doi.org/10.1074/jbc.M802249200
Yang, D., Gong, X., Yakhnin, A., & Roche, T. E. (1998). Requirements for the Adaptor Protein Role of Dihydrolipoyl Acetyltransferase in the Up-regulated Function of the Pyruvate Dehydrogenase Kinase and Pyruvate Dehydrogenase Phosphatase. Journal of Biological Chemistry, 273(23), 14130–14137. https://doi.org/10.1074/jbc.273.23.14130
Yihan, L., Xiaojing, W., Ao, L., Chuanjie, Z., Haofei, W., Yan, S., & Hongchao, H. (2021). SIRT5 functions as a tumor suppressor in renal cell carcinoma by reversing the Warburg effect. Journal of Translational Medicine, 19(1), 521. https://doi.org/10.1186/s12967-021-03178-6
Yin, C., He, D., Chen, S., Tan, X., & Sang, N. (2016). Exogenous pyruvate facilitates cancer cell adaptation to hypoxia by serving as an oxygen surrogate. Oncotarget, 7(30), 47494–47510. https://doi.org/10.18632/oncotarget.10202
Zachar, Z., Marecek, J., Maturo, C., Gupta, S., Stuart, S. D., Howell, K., Schauble, A., Lem, J., Piramzadian, A., Karnik, S., Lee, K., Rodriguez, R., Shorr, R., & Bingham, P. M. (2011). Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. Journal of Molecular Medicine, 89(11), 1137–1148. https://doi.org/10.1007/s00109-011-0785-8
Zhang, N., & Palmer, A. F. (2011). Development of a dichloroacetic acid‐hemoglobin conjugate as a potential targeted anti‐cancer therapeutic. Biotechnology and Bioengineering, 108(6), 1413–1420. https://doi.org/10.1002/bit.23071
Zhang, S., Hulver, M. W., McMillan, R. P., Cline, M. A., & Gilbert, E. R. (2014). The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutrition & Metabolism, 11(1), 10. https://doi.org/10.1186/1743-7075-11-10
Zhang, S.-L., Hu, X., Zhang, W., & Tam, K. Y. (2016). Unexpected Discovery of Dichloroacetate Derived Adenosine Triphosphate Competitors Targeting Pyruvate Dehydrogenase Kinase To Inhibit Cancer Proliferation. Journal of Medicinal Chemistry, 59(7), 3562–3568. https://doi.org/10.1021/acs.jmedchem.5b01828
Zhang, S.-L., Hu, X., Zhang, W., Yao, H., & Tam, K. Y. (2015). Development of pyruvate dehydrogenase kinase inhibitors in medicinal chemistry with particular emphasis as anticancer agents. Drug Discovery Today, 20(9), 1112–1119. https://doi.org/10.1016/j.drudis.2015.03.012
Zhou, Z. H., Liao, W., Cheng, R. H., Lawson, J. E., McCarthy, D. B., Reed, L. J., & Stoops, J. K. (2001). Direct Evidence for the Size and Conformational Variability of the Pyruvate Dehydrogenase Complex Revealed by Three-dimensional Electron Microscopy. Journal of Biological Chemistry, 276(24), 21704–21713. https://doi.org/10.1074/jbc.M101765200
Zhou, Z. H., McCarthy, D. B., O’Connor, C. M., Reed, L. J., & Stoops, J. K. (2001). The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proceedings of the National Academy of Sciences, 98(26), 14802–14807. https://doi.org/10.1073/pnas.011597698