Oxygen diffusion from capillaries to tissue mitochondria
-
How does oxygen diffuse from capillaries to tissue mitochondria? Barriers and pathways
"Although simplified models have treated oxygen diffusion within tissue as occurring in a homogeneous medium (Krogh, 1929, cited in Longmuir, 1981), tissue is now well-known to be inhomogeneous. The inhomogeneity may be exaggerated where cellular structural alterations occur. Such changes are particularly evident in cancers, where the level of organization of the microvasculature and the tissue tends to diminish, the fraction of interstitial fluid tends to increase, and certain cell types may become more abundant relative to others (Vaupel, 2004; Multhoff and Vaupel, 2012; Ding et al. 2019a). Moreover, the number of connections among cells in tumours may decrease (Cavallaro & Christofori, 2001). Associated changes in the physicochemical properties of the tissue may contribute to hypoxia and may help explain spatial heterogeneities in oxygen distribution."
"Diffusive delivery becomes especially important under circumstances where vessels are occluded or sparsely distributed, or where convective oxygen delivery via red blood cells is compromised, as in sickle cell disease."
"Timely subcellular delivery of adequate, but not too much, oxygen is also crucial to normal cellular physiology (Swartz, 1973), primarily due to its function in oxidative phosphorylation as an essential substrate for mitochondrial cytochrome c oxidase."
"Oxygen has generally been assumed to diffuse readily and passively through the capillary endothelium and through surrounding cell bodies, on its path to consumption in tissue mitochondria. The non-polarity and high lipid-solubility of oxygen suggests that it should generally pass through lipid bilayers without difficulty."
"[..]lipid bilayers are inhomogeneous along the axis of permeation and show substantial variation in both the partition coefficient and the diffusion coefficient as a function of bilayer depth (Diamond & Katz, 1974)."
"[..]the bilayer-depth region with the greatest concentration of the permeant is usually the region presenting least resistance to permeation (Diamond & Katz, 1974)."
"For oxygen, the greatest resistances occur near the bilayer headgroups, where the phospholipid packing density is greatest (Diamond & Katz, 1974; Dotson et al. 2017)."
"Because the headgroup-associated barriers are generally rate-limiting for oxygen permeation through a lipid bilayer (Ghysels et al. 2017), experimental permeability estimates generally carry considerable uncertainty."
"[..]the average solubility of oxygen within lipid bilayers is greater than that in buffered water (Möller et al. 2005)."
"Membrane cholesterol reduces permeability and enhances channelling"
"Cholesterol is a membrane compositional parameter important in cancers (Ding et al. 2019b). Dysregulation of cholesterol homeostasis is a common phenotype in solid tumours and can lead to increased membrane cholesterol levels (Li et al. 2006; Brown, 2007; Ding et al. 2019b). Most normal tissues have cholesterol content in the range 30−45% of the total lipid molecules (Weiner & Feigenson, 2018), while 50% cholesterol is normal for the lipid portion of red blood cell membranes and eye lens fibre cells (Jandl, 1996 and others reviewed in Widomska et al. 2007b)."
"A substantial body of work has examined the influence of membrane cholesterol on oxygen permeability and diffusion pathway (Subczynski et al. 1991; Dumas et al. 1997, 1999; Widomska et al. 2007a; Raguz et al. 2011; Angles et al. 2017; Plesnar et al. 2018; Angles and Pias, 2020; Dotson et al. 2020; Wang et al. 2020). Recent simulation studies have contributed several key findings. First, the overall oxygen lipid–water partition coefficient is reduced by 20−35% in membranes incorporating high levels of cholesterol (above 38%; Dotson et al. 2017, 2020). Yet, the local oxygen solubility is enhanced by cholesterol near the bilayer midplane, between the lipid leaflets (Dotson et al. 2017). Regardless of lipid composition, the bilayer midplane is typically characterized by a free energy well at the midplane, which is deepened with incorporation of saturated phospholipids and high levels of cholesterol (Subczynski et al. 1991; Dotson et al. 2017; De Vos et al. 2018a; Ghysels et al. 2019). Namely, high cholesterol content appears to promote physical ‘channelling’ of oxygen molecules toward the midplane (Fig. 1B) and to favour lateral diffusion within the bilayer, in the low-density region between the lipid layers (Dotson et al. 2017; Pias, 2020)."
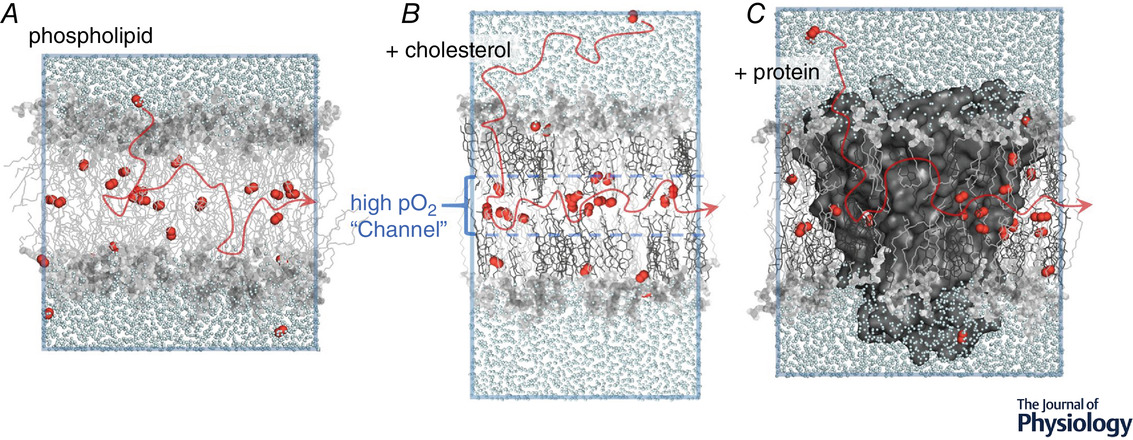
"The permeability coefficient of the high-cholesterol bilayers is also reduced relative to pure phospholipid, but the magnitude of reduction is not consistently predicted from the average membrane–water partition coefficient, as would be expected according to a simple application of Overton's rule (Dotson et al. 2017, 2020). The divergence of partition coefficient and permeability coefficient indicates that diffusion-related effects may modify the permeability where higher cholesterol levels are present. This finding is somewhat unexpected, as previous studies have indicated that the transbilayer diffusion coefficient is not likely to be altered by cholesterol content but is governed primarily by the depth-dependent local partition coefficient (Zocher et al. 2013; Dotson et al. 2017). For carbon dioxide in high-cholesterol bilayers, Overton's rule has been shown to hold locally, where the membrane is segmented into several regions with distinct partition coefficients (Zocher et al. 2013). For oxygen in high-cholesterol bilayers, the rate of bilayer crossing may additionally be modulated by lateral diffusion near the midplane, increasing the transit time of oxygen molecules as they cross one leaflet, diffuse laterally, then exit across the second leaflet (De Vos et al. 2018b). Though accurate estimation of the lateral, or ‘radial’, oxygen diffusion coefficient is lacking as a direct function of bilayer cholesterol content, it appears that cholesterol enhances lateral diffusion (Dotson et al. 2017; Plesnar et al. 2018; Ghysels et al. 2019)."
"Simulation studies indicate that lateral transport occurs due to a localized preference for radial movement, relative to transbilayer movement, in the interleaflet region (Ghysels et al. 2017, 2019). Diffusion coefficients for both transbilayer (normal) and lateral (parallel) movement are enhanced at the midplane, but the enhancement is greater for lateral diffusion."
"In phospholipid bilayer models lacking cholesterol, some O2 molecules have been found to travel along the midplane at distances up to 10 times the bilayer thickness, in the absence of a concentration gradient (at equilibrium; De Vos et al. 2018b). Further investigation will likely reveal longer lateral transit distances within high-cholesterol membranes, especially under non-equilibrium steady-state conditions with a directional driving force."
"In membranes incorporating protein, one might assume the permeability of the lipids themselves to be unchanged. However, the ESR and simulation studies both found the apparent permeability of the lipids to be reduced by the presence of protein, probably due to protein–lipid interfacial effects that have not been fully explained (Ashikawa et al. 1994; Dotson & Pias, 2018). These interfacial effects may involve the molecular dynamics of the lipids (Ashikawa et al. 1994), physical interaction of O2 molecules with the surface of the protein (Dotson & Pias, 2018), and possibly also surface characteristics of the protein, such as surface roughness or sidechain length."
"[..]the oxygen solubility in cytosol, and the effective diffusion coefficient, may be lower than generally understood. Both cytosolic and interstitial fluids are aqueous and have usually been modelled as pure water or saline. However, they are colloidal suspensions rich in solutes – including salts, metabolites, proteins and other macromolecules. The fluids are interspersed inside solid matrices (Moeendarbary et al. 2013) consisting of macromolecular crowders and cytoskeletal or extracellular matrix structures. The solubility of oxygen in these solute-rich fluids would likely diminish relative to water or saline. Oxygen solubility is known to decline with increasing salinity (Battino et al. 1983), and solvation of proteins and metabolites may cause additional ‘salting out’ of oxygen (Zander, 1976, cited in Longmuir, 1981; Skulachev, 1990). Indeed, low oxygen solubility in cytosol may confer a biological advantage, as a means of guarding oxygen-sensitive enzymes and other macromolecules from poisoning or oxidative damage (Longmuir, 1981)."
"**The effective diffusion coefficient of oxygen in the tissue milieu may be impacted by macromolecular crowding as well as the large number of dissolved metabolites. **Although macromolecular crowding is a more recently acknowledged phenomenon, structural and macromolecular components of tissue were already understood in the 1970s to influence the solubility and the diffusion coefficient of dissolved gases (Zander, 1975, cited in Vaupel, 1976). Indeed, Vaupel noted in 1976 that the water content of the diffusion medium alone was sufficient to predict the diffusion coefficient for oxygen. Specifically, he observed the O2 diffusion coefficient to decrease exponentially with decreasing water content, independent of viscosity effects. In comparison with its reference value in 100 wt% water, the O2 diffusion coefficient at 37°C was reduced by half in diffusion media containing 80 wt% water and further reduced to one-quarter the reference value at 65−70 wt% water (Vaupel, 1976)."
"[..]oxygen diffusion is rate-limited by the cytoplasm, in a manner dependent on the diffusive path length (related to cell shape) and tortuosity (related to haemoglobin crowding). Although membrane barriers are not ruled out, the study suggests that the dominant barrier to gas exchange is the cytoplasm, with an O2 diffusion coefficient approaching 70 μm2/s or less – nearly 30 times lower than the diffusion coefficient of oxygen in pure water (Richardson et al. 2020)."
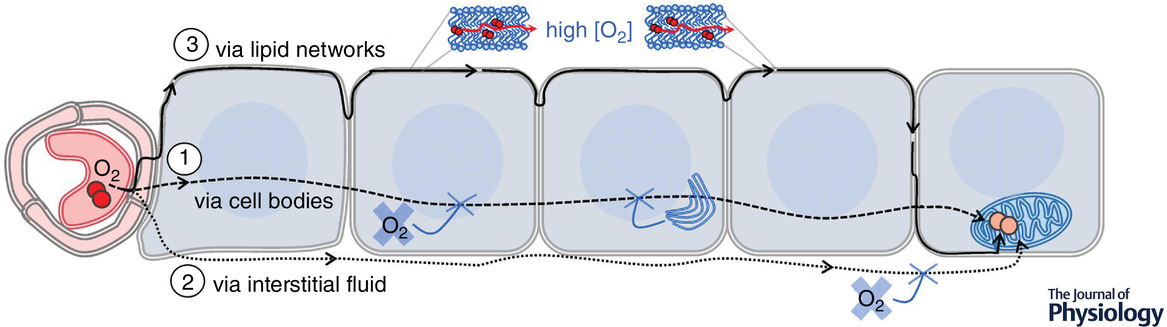
"Some oxygen must reach mitochondria several cell layers away. The preferential pathway is unknown, and three potential pathways are illustrated. Pathway 1: diffusion across cell bodies. Oxygen has often been assumed to diffuse across cell bodies, primarily via the cytosol. Opposing factors include low oxygen solubility in cytosol and intervening subcellular structures such as membranous organelles. Pathway 2: diffusion via interstitial fluid. A potential advantage of interstitial fluid over cytosol is the absence of organelles and associated membrane barriers. However, this pathway is disfavoured by low oxygen solubility in aqueous fluids and by limited extracellular space between cells. Pathway 3: diffusion via lipid networks. This route appears to be the preferential pathway for oxygen diffusion within tissue, due to greatly enhanced oxygen solubility in lipids and a locally enhanced diffusion coefficient near the midplane of lipid bilayers (‘channelling’, as in Fig. 1). The number of membrane-associated barriers encountered on this pathway would be lower than the number encountered in crossing cell bodies. Membrane proteins may tend to reduce the rate of diffusion within lipid bilayers."
"In diffusing across cell bodies, oxygen would encounter many membrane-associated barriers. While the plasma membrane alone would be unlikely to limit the diffusion rate, the cellular milieu is exceedingly rich in organellar membranes, especially those of the endoplasmic reticulum and mitochondrial networks. Organellar compartments, cytoskeletal structures and macromolecules would present additional barriers. Therefore, oxygen diffusing across cell bodies would encounter resistance from the low-solubility aqueous cytoplasm, from multiple membranes, and from other cellular structures as well as macromolecular crowders. While the interstitium (pathway 2) lacks some of the obstacles, it, too, is rich with fibrous extracellular matrix structures and soluble proteins. The cells of healthy tissues are interconnected through junctions, leaving narrow and confined spaces for potential oxygen diffusion. Along either pathway 1 or 2, steering around the obstacles would substantially lengthen the diffusion path."
"Pathway 3, through networked lipids, is supported by the preferential solubility of oxygen in lipids versus water, anisotropically enhanced lateral diffusion of oxygen near the midplane of lipid bilayers, and continuous networks of membranes and lipid droplets within and among cells of healthy tissues. Compared with water or aqueous buffer, oxygen solubility is greater by a factor of 3−5 for whole membranes (Möller et al. 2005, 2016; Möller & Denicola, 2018) and by a factor of 10 or more near the bilayer midplane (Al-Abdul-Wahid et al. 2006). Oxygen has been reported by Longmuir, citing Krogh (1929), to be ‘virtually insoluble in cytosol’, and Longmuir's own measurements in cells indicate a factor of 25 enhancement of oxygen solubility in localized, lipid-based ‘channels of high solubility’ (Benson et al. 1980; Longmuir, 1981; Longmuir et al. 1981)."
"Along pathway 3, the resistance of low-solubility aqueous fluids would be reduced by shortening the distance travelled within them. Inside membranes, the local diffusion coefficient is enhanced anisotropically, to favour lateral diffusion (Fig. 1; Ghysels et al. 2017; De Vos et al. 2018b). The presence of transmembrane proteins, especially within ordered microdomains (rafts), could present physical obstacles to slow diffusive progress. However, even ordered raft lipids may facilitate channelling along the midplane (Ghysels et al. 2019). In such a lipid-dominated diffusion scheme, the lipids would serve primarily to accelerate oxygen diffusion, by reducing the path-length of diffusion through resistant aqueous fluids and by providing ‘channels’ of high solubility and enhanced diffusion coefficient. Experimental evidence of accelerated oxygen diffusion via networked lipids has been reported in lung surfactant (Olmeda et al. 2010)."
"Figure 3 illustrates potential lipid-based diffusion pathways within cells, as proposed by various researchers. The figure highlights the low solubility of oxygen in cytosol and visualizes accelerated diffusion through the endoplasmic reticulum, mitochondrial networks and neutral lipid droplets. Sufficiently fluid neutral lipid droplets may facilitate oxygen diffusion (Desaulniers et al. 1996; Sidell, 1998), while highly ordered ones may present obstacles."
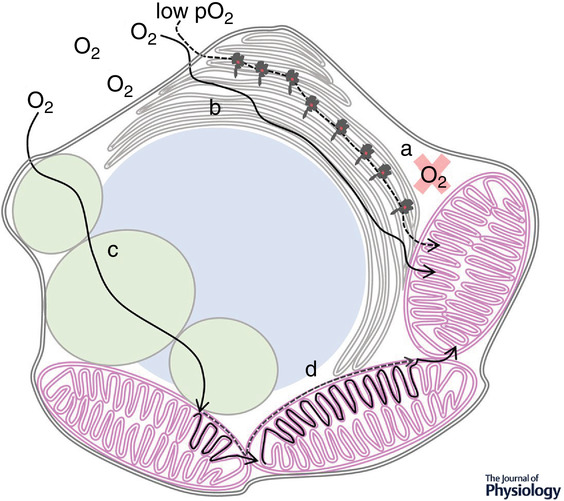
"Oxygen availability depends on the path of its diffusion from blood capillaries to sites of consumption within tissue. Cumulative evidence suggests that the molecular structures of tissue, especially cellular membranes, can influence the rate of oxygen diffusive delivery. Lipids may act primarily as accelerants, to overcome aqueous barriers generated by low oxygen solubility and, thus, high resistance. Minimizing the thickness of aqueous layers may promote rapid ‘channelling’ of oxygen along high-solubility pathways. The solubility is especially high near the midplane of lipid bilayers, between the lipid leaflets, and cholesterol enhances the channelling effect. The oxygen diffusion coefficient is, likewise, enhanced near the midplane, in an anisotropic manner favouring lateral diffusion between the lipid layers. Both cholesterol and transmembrane proteins reduce oxygen transbilayer permeability and may modulate the rate of oxygen transit across cells and tissues if the preferential diffusion pathway is lipid-dominated."