Pantothenic acid forms in foods
-
"[..]CoA is the major pantothenic acid-containing compound in yeast, pig liver and peas, but that it was not found, on the other hand, in the avocado, carrots, French beans and salmon. In the four other non-supplemented foodstuffs studied (chicken egg, chicken meat, spinach, lentils), the percentage of pantothenic acid released from CoA is included between 20 and 30%. These results confirm that CoA is effectively the major pantothenic acid-containing compound in animal liver and yeast as has already been mentioned by Brown [29] and Gonthier et al. [6], but they seem partially to refute the general but unsupported allegation of Ball [30], according to which the majority of naturally occurring pantothenic acid would be in the form of CoA."
Liver Coenzyme A Levels in the Vitamin B6-Deficient Rat
"Liver CoA concentration in the rat was decreased 50% by vitamin B6 deficiency and 66% by pantothenic acid deficiency. The decrease caused by vitamin B6 deficiency was independent of food intake and was not remedied by cystine supplementation of a 20% casein diet."
-
- Coenzyme A (CoA) functions as a key metabolic cofactor under physiological conditions.
- CoA switches to be a major cellular antioxidant under oxidative or metabolic stress.
- CoAlation is a widespread and reversible protein modification in cellular response to oxidative stress.
- The CoAlation/deCoAlation cycle is enzymatically controlled.
- The pattern of protein CoAlation changes in pathologies associated with oxidative stress.
"Under oxidative or metabolic stress, CoA was found to use the thiol group for covalent modification of oxidized cysteine residues, protecting them from irreversible overoxidation and facilitating the antioxidant response."
"The pKa value for the CoA thiol group is high (9.83), which is determined by its position at the tip of the pantetheine tail [41]. Therefore, CoA exists predominantly in its unreactive thiol form at physiological pH, which protects it from auto-oxidation and oxidation to the sulfenic acid state."
"To be engaged in the nucleophilic attack, the CoA thiol group has to be activated to a thiolate state by enzyme(s) which can reduce the pKa value of the CoA thiol and facilitate covalent modification of cellular targets. The proposed mechanism was reported for GSH in complex with glutathione S-transferase-pi [42]. The identity of CoA transferase(s) remains to be revealed and their involvement in redox regulation investigated.
Furthermore, CoA has a good capability to buffer oxidative stress in cells as its redox potential (−234 mV) is near to that of glutathione (−240 mV) which is the best-studied LMW thiol in the antioxidant response [43,44]."
"We hypothesize that CoA functions as a key metabolic integrator under physiological conditions, but switches to be a major cellular antioxidant in response to oxidative or metabolic stress."
-
Very interesting, thank you for providing all the research info!
-
Lathering the skin with D-panthenol (or dexpanthenol) may lead to local overconsumption of NAD+.
Nutrient Metabolism: Structures, Functions, and Genes (978-0-12-387784-0)
"CoA synthesis uses pantothenate, cysteine, one adenylate, three phosphates, and the energy of six high-energy phosphates from ATP (Figure 10.43). Significant amounts of pantothenate are generated from pantetheine through the action of pantetheine hydrolase (EC3.5.1.-), which is expressed in many tissues. Panthenol and panthenal may also be converted to a limited extent into pantothenate by alcohol dehydrogenase (EC1.1.1.1) and aldehyde dehydrogenase (EC1.2.1.3)."
- Panthenol -(ADH)→ Panthenal -(ALDH)→ Pantothenic acid
⠀
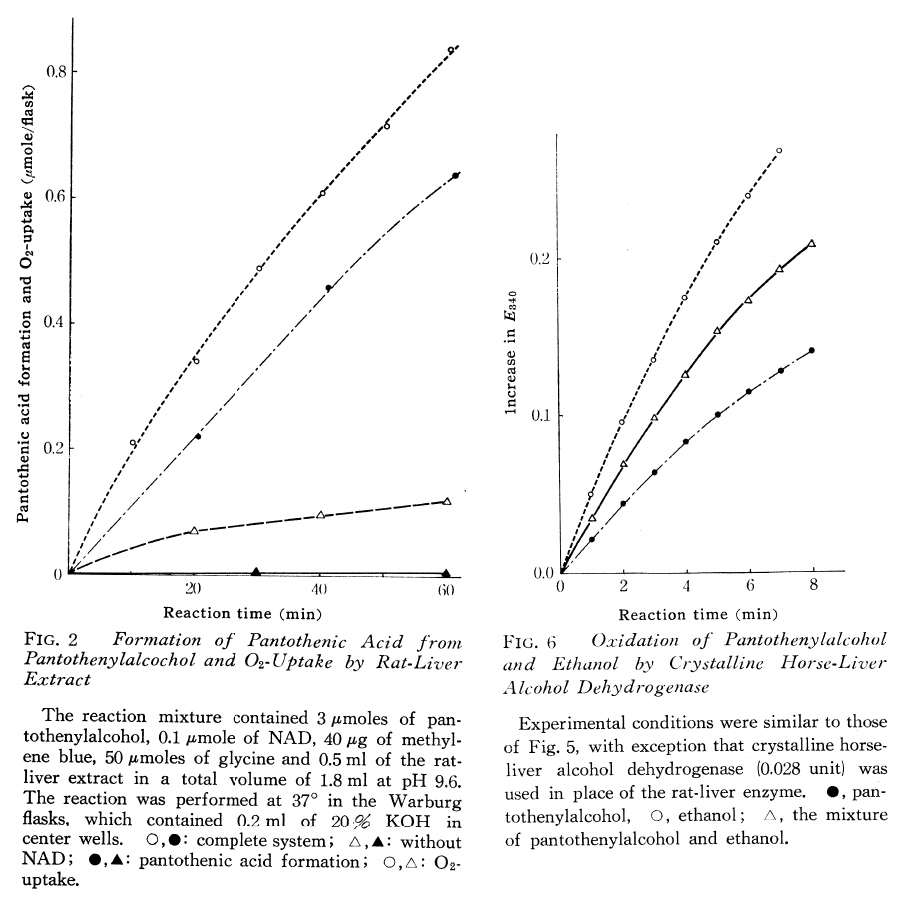
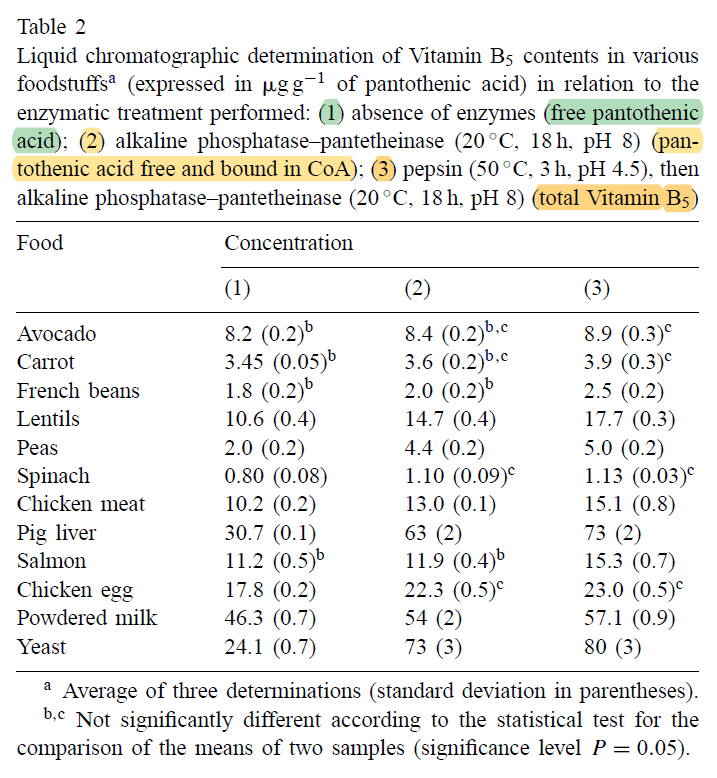
Enzymatic Conversion of Pantothenylalcohol to Pantothenic Acid