Cobalamin (B12)
-
Photodegradation of cobalamins in aqueous solutions and in human blood
"Human plasma contains a variety of cobalamins (
Fig. 1) including methylcobalamin (MeCbl, 64%), adenosylcobalamin (AdCbl, 15%), hydroxocobalamin (OHCbl, 12%) and cyanocobalamin (CNCbl (sometimes called vitamin B12), 9%) [3].""It is known that intracellular cobalamins react rapidly with nitric oxide and superoxide [21,30-32], which can be generated in skin or blood during exposure to UVA [33,34]. Additionally, skin and blood contain many endogenous photosensitizers (porphyrins, flavins) absorbing in the UVA region, and exposure to UVA leads to the generation of reactive oxygen species [35,36]. Singlet oxygen, superoxide anions, hydrogen peroxide and triplet-state riboflavin radicals are generated under exposure of riboflavin (RF) to UVA [37]. Reactive oxygen species are quenched by antioxidants in human skin which may, due to this, indirectly be degraded. Cobalamins may also quench singlet oxygen and hydrogen peroxide, and it may photodegrade indirectly. However, the indirect photodegradation of cobalamins due to their antioxidant properties have not been studied."
"In the present study we have investigated the photodegradation of four major forms of vitamin B12 (MeCbl, AdCbl, OHCbl and CNCbl) under UVA exposure in aqueous solutions at physiological pH by absorption spectroscopy. The degradation of OHCbl exposed to UVA in the absence and presence of the endogenous photosensitizer RF was investigated. Serum vitamin B12 concentrations before and after summer were measured in four patients with psoriasis."
"All studied cobalamins degrade under UVA exposure in aqueous solutions at physiological pH (
Fig. 4-5). OHCbl was found to be the most stable cobalamin under our experimental conditions (Fig. 4A and 5A).""However, OHCbl is an antioxidant, and may degrade indirectly in the presence of reactive oxygen and nitrogen species generated under UVA exposure. The presence of endogenous photosensitizer RF during UVA exposure increased the degradation of OHCbl (
Fig. 7, 8A). RF and nicotinamide enhance the degradation of CNCbl in aqueous solution [24]. CNCbl is phototransformed to OHCbl [20,23]. It is possible that after entering a cell, OHCbl is transformed back to AdCbl and MeCbl, and thus may retain the biological activity of the vitamin, even in the case that the active forms will first be degraded in vivo. Nevertheless, high doses of solar radiation (or artificial UV radiation, e.g. sunbeds) may, at least theoretically, diminish the effectiveness of vitamin B12 and impair its organic functions. However, it is not simple to measure the changes of MeCbl, AdCbl or OHCbl concentrations in blood.""AdCbl and MeCbl were extremely unstable and photodegrade in seconds (
Fig. 4C-D and 5C-D). They were photoconverted to OHCbl (Fig. 6), which is in agreement with the earlier observations by others [18,21]. MeCbl was around 3-fold more sensitive to UVA radiation than AdCbl (Fig. 5CD).""The degradation rate of OHCbl in the presence of RF was around 3 times faster than alone (
Fig. 8A), while the degradation rate of RF was not influenced by the presence of OHCbl (Fig. 8B).""The vitamin B12 status is most often assessed by measuring serum or plasma total cobalamins. Due to the fact that OHCbl is quite photostable, it is not surprising that seasonal variations of serum vitamin B12 were not observed in healthy people [47,48]. Our preliminary results on the influence of solar radiation on vitamin B12 levels in psoriasis patients demonstrate that sun exposure during a summer in Norway was not able to destroy the vitamin, while the same exposures increased the levels of 25-hydroxyvitamin D in serum by 66% (
Table 1). This means that significant doses of UV radiation reached tissues below the stratum corneum. We also found that serum homocysteine levels were not significantly increased (indirect measurement of vitamin B12 and folate changes). However, longer exposure times in sunlight at lower latitudes or in sunbeds (which have several times higher UVA fluence rates than found in solar radiation [49,50]) may lead to increased plasma homocysteine concentrations due to the photodegradation of vitamin B12 but not to any photodegradation of folate, as was observed in patients with psoriasis by Osmancevic et al. [16].""Cobalamins circulate in blood bound to two transport proteins, haptocorrin and transcobalamin [51-53]. The major part of cobalamins are bound to haptocorrin (80-96%) [53], which has an unknown function, but it has been proposed that haptocorrin serves to protect cobalamins [22,51]. It was demonstrated that haptocorrin protects MeCbl from 442 nm photodegradation [22]. Calculations show that this mechanism may be of significance at light doses penetrating the skin, and act to protect cobalamins [22,51]."
"It is interesting to note that vitamin B12 deficiency may cause cutaneous hyperpigmentation [56-61]. The dominant mechanism of hyperpigmentation is an increase in melanin synthesis [62]. This clearly demonstrates that vitamin B12 is involved in melanin synthesis, and that vitamin B12 might have had a more important evolutionary role than folate (which absorbs only in the UVB region) in the development of different skin colours. American and British black-skinned persons have significantly higher serum vitamin B12 concentrations than white persons have [63-66]. The ethnic differences in vitamin B12 levels probably arise from combinations of hereditary and acquired properties unrelated to cobalamin intake, storage, or deficiency [67]."
-
One claim in this guy's article that has basis:
"I discovered how B12 promotes healthy gastrointestinal microbial activity[.]"
I think that it originated from here, where authors suggest..
"Based on recent studies of the role of corrinoids in the human gut, we propose that targeted manipulations of the gut microbiota could be achieved by altering the levels of specific corrinoids. This hypothesis is based on the following observations.
- First, over 80% of sequenced human gut bacteria are predicted to use corrinoids, but less than 25% have the genetic capacity to synthesize these molecules (Degnan et al., 2014);
- Second, human fecal samples have been found to contain up to eight distinct corrinoids (Allen and Stabler, 2008).
- Third, only a fraction of the corrinoids available in a microbial community can be used by a given organism because corrinoid-dependent enzymes have native specificity for their preferred cofactors (Keller et al., 2013; Mok and Taga, 2013; Yi et al., 2012).
- Fourth, corrinoids other than cobalamin are poorly recognized by human intrinsic factor, the key protein required for transport of cobalamin from the lumen of the small intestine.
- Finally, corrinoids are synthesized exclusively by bacteria and archaea, unlike many other vitamins that are also prevalent in plant-based dietary components (Roth et al., 1996).
Thus, because different bacteria require distinct groups of corrinoids, we hypothesize that microbial communities can be manipulated by altering the levels of particular corrinoids."
You can find it on the Garrey Smeat Forum.
The authors above adopted a more conservative tone, similar to that of the group who did a comprehensive review on the topic:
Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review
"Findings from in vitro, animal, and human studies suggest that vitamin B-12 may be associated with changes in bacterial abundance. While evidence from in vitro studies indicates that vitamin B-12 may increase alpha-diversity and shift gut microbiome composition (beta-diversity), results from animal studies and observational human studies were heterogeneous."
They added:
"Potential mechanisms Modulation of the gut microbiome by vitamin B-12 or other nutrients in one-carbon metabolism may occur via several potential mechanisms. The impact of vitamin B-12 on the gut microbiome may be explained by vitamin B-12–dependent enzymes and riboswitches (14, 18); however, the mechanisms that link vitamin B-12 and the human gut microbiome have not been fully established. One-carbon metabolism. The role of other nutrients in one-carbon metabolism may provide further insights into the links between vitamin B-12 and the gut microbiome. For example, SAM production (i.e., a methyl donor and product of one-carbon metabolism) can be used for DNA methylation in bacteria to alter gene expression (100) or in mucosal cells, which could impact the intestinal environment (99, 101, 102). SAM is also a substrate for production of metabolites used in communication between bacteria (i.e., quorum sensing) (103, 104). Through B-vitamin sharing among bacteria in the gut microbiome (20), bacteria may benefit from an abundance or stability of B-vitamin availability in the gut environment (105). Additionally, products of B-vitamin metabolism may be used by other bacteria in the gut (20, 105, 106), which may offer competitive advantage to some bacteria without B-vitamin–dependent enzymes. Other mechanisms. Choline can be metabolized by bacteria in the gut microbiome to produce trimethylamine (82), which may decrease choline availability for the host (107) and limit production of SAM. Additionally, niacin may have a role in decreasing bacterial endotoxin production and improving intestinal barrier function (108, 109), and Steinert and colleagues (92, 110) hypothesized that riboflavin may reduce oxidative stress via NADH-redox reactions in bacteria."
"Findings from in vitro and animal studies suggest that specific cobalamin forms may differentially impact alpha-diversity and beta-diversity and bacterial abundance. Although in humans, all cobalamin forms are interconverted and transported within the cell (64), different forms of cobalamin may have distinct roles in bacteria metabolism. Differences in cellular transport among forms is unlikely, as the predominant transporter, an ATP-binding cassette (ABC)-type BtuFCD transport system, recognizes the lower ligand of cobalamin that is shared among all forms (18). In contrast, cobalamin forms may differ in enzyme affinity (i.e., adenosylcobalamin-dependent enzymes) or ability to control gene expression through riboswitches—regulatory elements of mRNA."
"Most cobalamin riboswitches are thought to be adenosylcobalamin-dependent, as demonstrated in some model bacterial species (65–67), but some Escherichia coli riboswitches have over 500 times higher affinity for methylcobalamin and aquocobalamin, compared with adenosylcobalamin (68). Zhu et al. (26) found that cyanocobalamin improved growth and increased Shiga toxin production of E. coli compared with methylcobalamin, whereas Lactobacillus reuti had similar growth with both cobalamin forms. Cyanocobalamin and methylcobalamin also had different effects on enzyme activity, and cyanocobalamin had greater inhibition of riboswitch expression. In contrast, in the Propionibacterium strain UF1, cyanocobalamin, methylcobalamin, hydroxycobalamin, and adenosylcobalamin had similar regulation of genes involved in vitamin B-12 biosynthesis (69). As genomic studies elucidate mechanisms of gene regulation in bacteria, in vivo studies are needed to determine how different cobalamin forms impact the bacterial community."
The positive outcomes are uncertain and, just as with various other nutrients, it's possible for B12 to be a virulence factor.
When only good effects are anticipated, people are tempted to exaggerate.
"Daily oral cobalamin supplements can contain doses that far exceed (as high as 10,000 μg/tablet) the recommended daily amount of 2.4 μg/day in humans. High exposures are required when normal absorption is impeded and are generally considered safe. The practice of over-supplementing cobalamin to ensure adequate absorption is supported by the fact that no upper limit has been set for B12 supplementation. A study in C57BL/6J mice using a dose comparable to a 5000 μg tablet (30.46 μg/day) for humans found a depletion in Bacteroides but no change in cecal short-chain fatty acids (SCFAs) or markers of dextran sulfate sodium (DSS)-induced colitis with B12 treatment, including colon length and fluorescein isothiocyanate-dextran permeability test [15]. Since competition exists between enteric pathogens and commensal microbes for B12 sequestering and sharing, we hypothesized that excessive cobalamin supplementation alters the gut microbiota’s functional activity, creating a favorable environment for pathogen colonization and pathogenesis. Due to the fact that no upper limit has been determined for B12, we supplemented a megadose of B12 to mice in drinking water and challenged with Citrobacter rodentium, a natural mouse-specific pathogen that mirrors the attaching and effacing pathology seen in human EHEC infections [16]. We evaluated the direct and indirect impact of oral B12 supplementation on the gut microbiota and ability of the host to resist enteric pathogen colonization and pathogenesis."
"According to water intake, B12 supplemented in drinking water at 10 μg/ml and 40 μg/ml were estimated to reach a total dose of 30 μg and 120 μg per mouse a day, respectively. The human equivalent dose for the cyano- and methyl-cobalamin drinking water used in these studies is ~5000 μg/day (CNCbl10 & MeCbl10) and ~25,000 μg/day (CNCbl40 & MeCbl40), which was calculated using the standard human equivalent dose parameters [18]. A megadose was used in the present study to push the phenotype and ensure high B12 levels were maintained in the gut to disrupt competition, cross-feeding and microbe-host interactions related to B12 utilization by the gut microbiota. These levels were considered feasible within the human population, where 10,000 μg B12 capsules are available and would be taken as a single bolus. Comparing B12 supplementation between humans and mice using a multiplicity factor of their respective recommended dietary allowance (RDA) shows that a dose of 5000–10,000 μg tablet per day in an adult human is equal to 2083–4167 × their RDA of 2.4 μg/day, whereas mice receiving a 120 μg/day in the present study is equal to 2400 × their RDA. The RDA of B12 for mice is approximately 0.05 μg/day and is based on a diet containing 10 μg of B12 per kilogram that was deemed adequate for mice [19]."
"In the present study, we showed that excessive B12 concentrations in the gut can induce a dysbiosis that promotes C. rodentium colonization and pathogenesis in mice. The B12-induced dysbiosis can be characterized by the decrease in members belonging to Clostridia vadinBB60 and Lachnospiraceae NK4A136 groups, along with an increased abundance of Parasutterella in the cecum and colon. The changes to the gut microbiota from B12 supplementation stimulated the production of the IL-12/23p40 subunit and IL-17A cytokines in the colon and opened the gut niche for C. rodentium colonization without any obvious tissue damage prior to pathogen challenge. In general, oral B12 supplementation can contribute to a dysbiosis that promotes low-grade inflammation and a virulent C. rodentium population regardless of cobalamin type. Through changes to the microbiota, a B12-saturated gut environment shifts the metabolism of C. rodentium from avirulent to virulent as determined by the decrease in glucose enzyme activity and increase in virulent gene expression."
As for seafood broth containing free B12 and the broth being superior to supplemental form in correcting a deficiency, it's tricky.
Broth from Canned Clams Is Suitable for Use as an Excellent Source of Free Vitamin B12
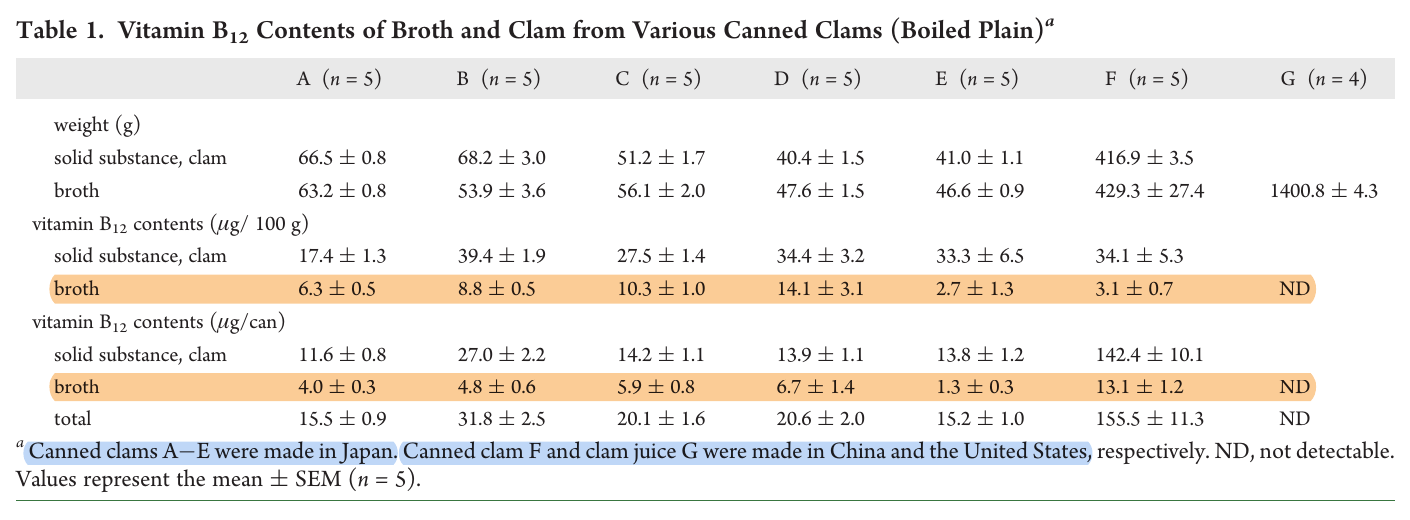
Not all B12 that's extracted is free (or perhaps in the desired form). They point out that it may be 70% of the cobalamin present in the broth, lowering its content to no more than 10 mcg free B12 per can.
We have other sources reporting higher amounts:
Characterization of vitamin B12 compounds from marine foods
"Vitamin B12 is synthesized by only certain bacteria and archaea but not by animals or plants. In marine environments, bacterial vitamin B12 is transferred and concentrated into fish and shellfish bodies by plankton in the marine food chain. Moreover, marine macrophytic red algae, Porphyra spp. specifically contain substantial amounts of vitamin B12, due to microbial interaction. Although some meats or viscera of edible fish and shellfish are excellent sources of biologically active vitamin B12, an inactive corrinoid, pseudovitamin B12, was found in some edible shellfish using liquid chromatography/electrospray ionization–tandem mass spectrometry. To prevent elderly people from developing vitamin B12 deficiency due to food protein-bound vitamin B12 malabsorption, we present a survey of marine foods containing free vitamin B12. The results of our study suggest that bonito and clam extracts (or soup stocks), which contain considerable amounts of free vitamin B12 are useful not only as seasonings and flavorings but also as excellent sources of free vitamin B12."
"Various types of fish soup stocks and extracts are manufactured for use as seasonings or flavoring. A certain extract made from raw muscle of bonito contains the highest free B12 (approximately 41 μg/100 g wet weight) among various fish extracts tested (Nishioka et al. 2008). Moreover, short-necked clam extract contains substantial amounts of free B12 (approximately 132 μg/100 g wet weight) (Ueta et al. 2010). The broth of canned clams also contains free B12 (approximately 14.1 μg/100 g wet weight) (Ueta et al. 2011b ⇈). Salted and fermented salmon kidneys (called "Mefun"), which have a delicate flavor and are popular only in Japan, contain substantial amounts of B12 (approximately 328 μg/100 g wet weight) (Adachi et al. 2005), most of which are derived from free B12. These results suggest that these fish and shellfish products are excellent sources of free B12 for preventing B12 deficiency among elderly persons with food-bound B12 malabsorption."
Based on the previous material, they picked the highest content of all options and treated it entirely as free B12. Maybe they did the same with other values, but I haven't checked.
If the person can't digest food properly, the poor liberation of B12 would only be one of the consequences of a greater concern, that possibly involves a more generalized state of malnutrition.
In addition, free B12 doesn't guarantee sufficient absorption, the problem can be compounded by not being able to synthesize the protein needed to bind it (to maximize absorption) or an inability to absorb the complex after it forms in the gut. These are the majority of cases that are prone to progress to a serious deficiency, increasing the reliance on the passive process, that doesn't depend on protein complexation in the gut.
How I treat cobalamin (vitamin B12) deficiency
"In more limited absorptive disorders, such as malabsorption confined to food-bound cobalamin (FBCM),[27] impaired cobalamin release from food limits but does not incapacitate the IF system, which also presumably continues reabsorbing biliary cobalamin. Some forms of FBCM remit after antibiotics.[28,29] The rate of cobalamin depletion is unknown but is probably many years slower than in IF-related disorders (Table 2). If the underlying disorder is nonmalabsorptive, such as dietary insufficiency, clinical consequences are delayed even longer[30,31] because absorption and reabsorption are intact and because intake fluctuates. Only in rare disorders of cellular utilization do consequences appear rapidly (Table 2)."
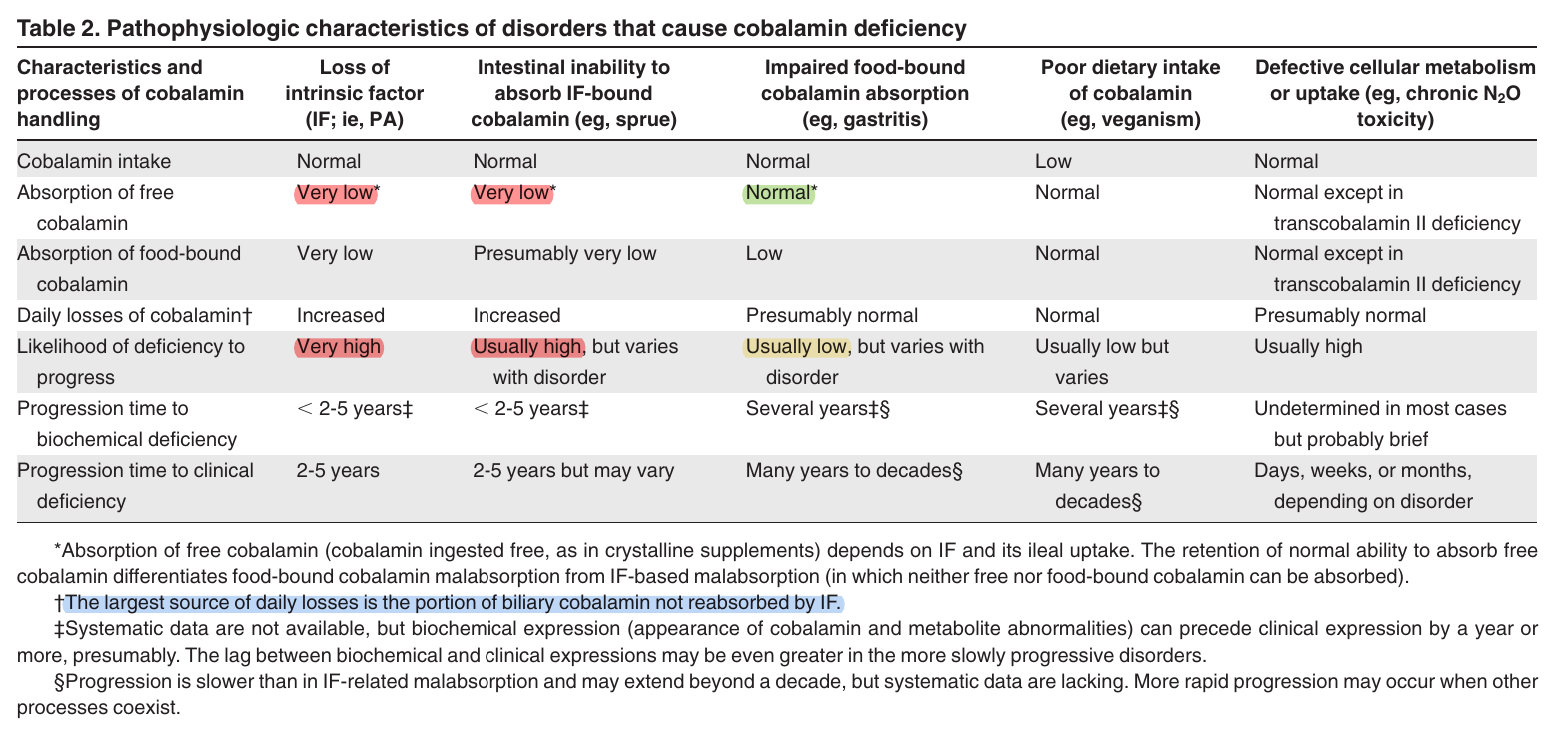
"Not surprisingly, most adults who come to medical attention with clinically expressed cobalamin deficiency are those with IF-related malabsorption. Savage et al[32] reported that 94% of patients with clinically expressed deficiency had IF-related malabsorption, such as PA or sprue; only 1% were vegetarians or had (presumptive) FBCM."
So, if we're communicating with people who are deficient, they might have these compounding factors present.
Supposing that the person finds seafood appetizing for frequent consumption and doesn't discard the arguably off-putting broth when products are canned, a generous content of 100 mcg free B12/100 g of broth would fall within a questionable therapeutic range. It's rare to find guidelines for B12 repletion with doses around 100 mcg, for being too high for the easily-saturable and (possibly) dysfunctional active process, and too low to compensate for the inefficiency of the passive absorption.
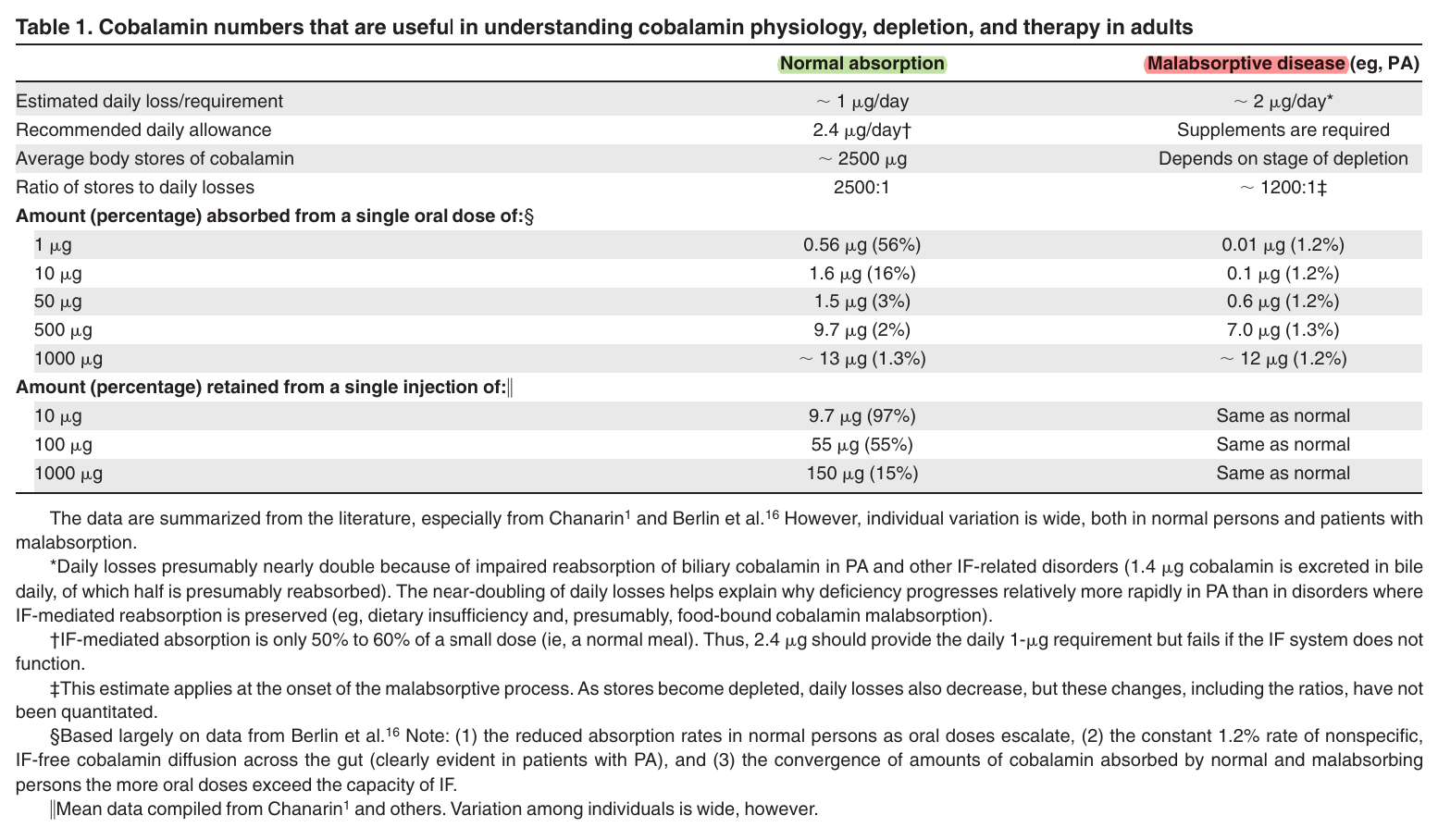
The graphed values:
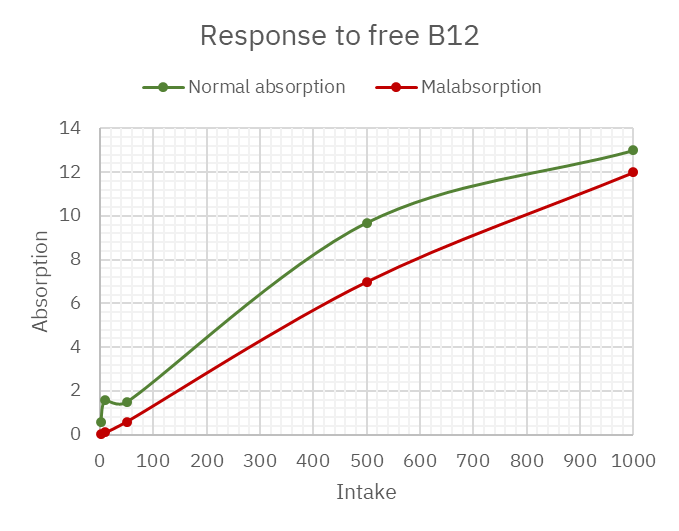
The content of about 10 mcg free B12 in broth per can would barely tickle someone with malabsorption (the majority who are prone to deficiency). But when the only issue is releasing it from food and smaller amounts of free B12 are enough to avoid the problem, some hours are needed to renew the dose when counting primarily on the active process.
Dietary Reference Intakes for Vitamin B12 | Institute of Medicine
"The second of two doses of B12 given 4 to 6 hours apart is absorbed as well as the first (Heyssel et al., 1966)."
Making it impractical to depend on a food for this dose renewal.
Altogether, if you're deficient in B12, rather than relying on clam soup to get you covered, it's preferable to skip to the superior option: supplements. These allow the selection of form, amount, and timing depending on needs. Some people don't respond unless they take 500 mcg or more.