PPAR Gamma Mediates High-Fat Diet-Induced Adipocyte Hypertrophy and Insulin Resistance
-
PPARγ is playing major role in development in metabolic diseases. Biological differences in expression of PPARγ could be the important part when it comes to explaining susceptibility to metabolic diseases.
"... The expression of PPARγ has been shown to be sufficient to initiate adipogenesis in exponentially growing fibroblast cell lines, demonstrating a critical role for this receptor in the regulation of adipocyte differentiation. Embryonic fibroblast (EF) cells from mice lacking both C/EBPβ and C/EBPδ failed to express PPARγ and C/EBPα and were unable to differentiate in response to hormonal stimulation. EF cells from mice lacking C/EBPα failed to express PPARγ and were also unable to differentiate into adipocytes. Recently, ADD1/SREBP1 and PPARγ were reported to cooperate via the production of PPARγ ligand and stimulation of PPARγ expression by ADD1/SREBP1."
"Thiazolidinedione (TZD), a new class of antidiabetic drugs that increase insulin sensitivity, can directly bind and activate PPARγ and can stimulate adipocyte differentiation. We have previously shown that treatment of Zucker fa/fa rats with TZD caused an increase in the number of small adipocytes and a concomitant decrease in the number of large adipocytes that had produced an excess amount of TNFα and free fatty acids, thereby ameliorating insulin resistance. These results led us to propose that stimulation of PPARγ with potent synthetic agonists such as TZD may result in adipocyte differentiation and a consequent increase in the number of small adipocytes, thereby rendering the body more sensitive to insulin."
"PPARγ+/− showed normal weight gain and insulin sensitivity under a standard diet. To determine the role of PPARγ in HF diet–induced obesity and insulin resistance, we studied body weight gain and white adipose tissue mass of wild type and PPARγ+/− under either a high-carbohydrate (HC) diet or a HF diet. Body weight gain under a HC diet for 15 weeks was not distinguishable between wild type and PPARγ+/−. Under a HF diet, wild type gained significantly more body weight than that under a HC diet. In contrast, PPARγ+/− had little weight gain under a HF diet Gross appearance at sacrifice after 16 weeks of a HF diet revealed that fat mass in wild type under a HF diet was higher than that in both types of mice under a HC diet. PPARγ+/− were protected, at least in part, from the increased fat mass under a HF diet Moreover, marked fatty liver observed in wild type under a HF diet was again in part prevented in PPARγ+/−"
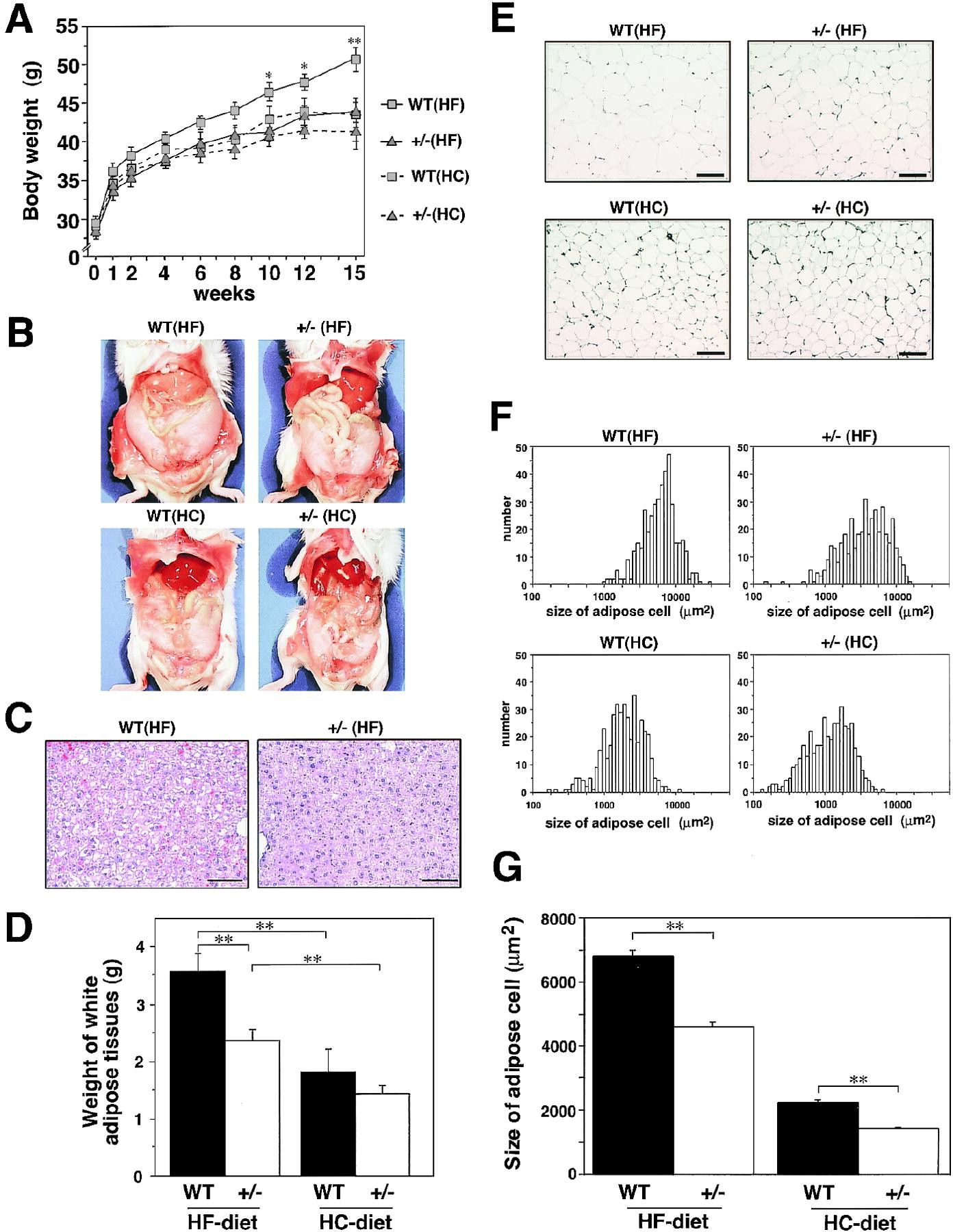 .
."PPARγ appears to facilitate energy storage under a HF diet in part by inhibiting expression of the leptin gene in adipocytes, the major hormonal regulator of energy homeostasis. It seems that PPARγ serves as a typical thrifty gene that provided a survival advantage in times of fast. However, in times of feast and sedentary life-style, such as today in Western countries and Japan, the two alleles of PPARγ appear to provide a survival disadvantage, causing an epidemic of hypertrophic obesity leading to serious obesity-linked morbidity and mortality. Finally, the identification of a novel role for PPARγ in obesity and insulin resistance should provide insight into the establishment of novel therapeutic strategies, such as PPARγ antagonist, for obesity and obesity-linked insulin resistance."