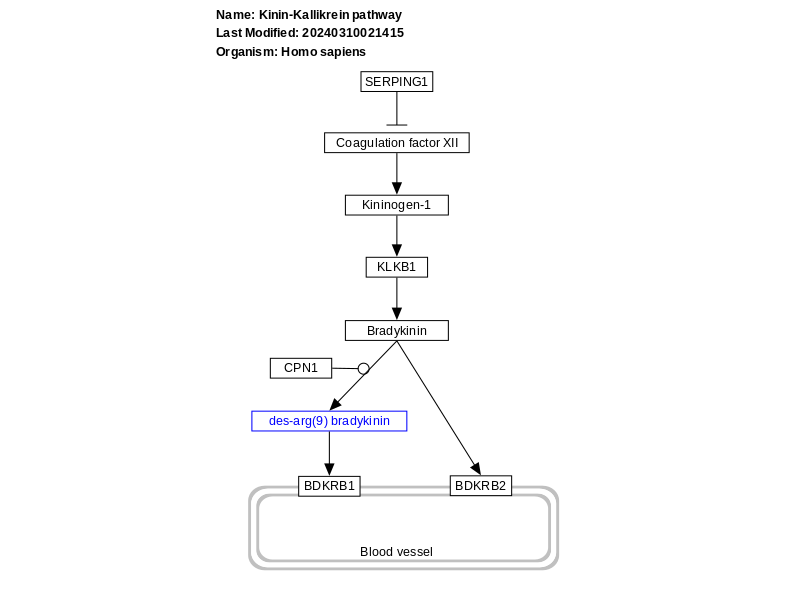
Bradykinin: A Fundemantal Player in the Stress Cascade
-
Bradykinin:
- increases nitric oxide
- increases inflammatory cytokines
- increases pain hypersensetivity
- causes mast cell degranulation
- causes vascular leakage
- causes edema
- exacerbated by estrogen
RAAS and bradykinin pathways in COVID-19
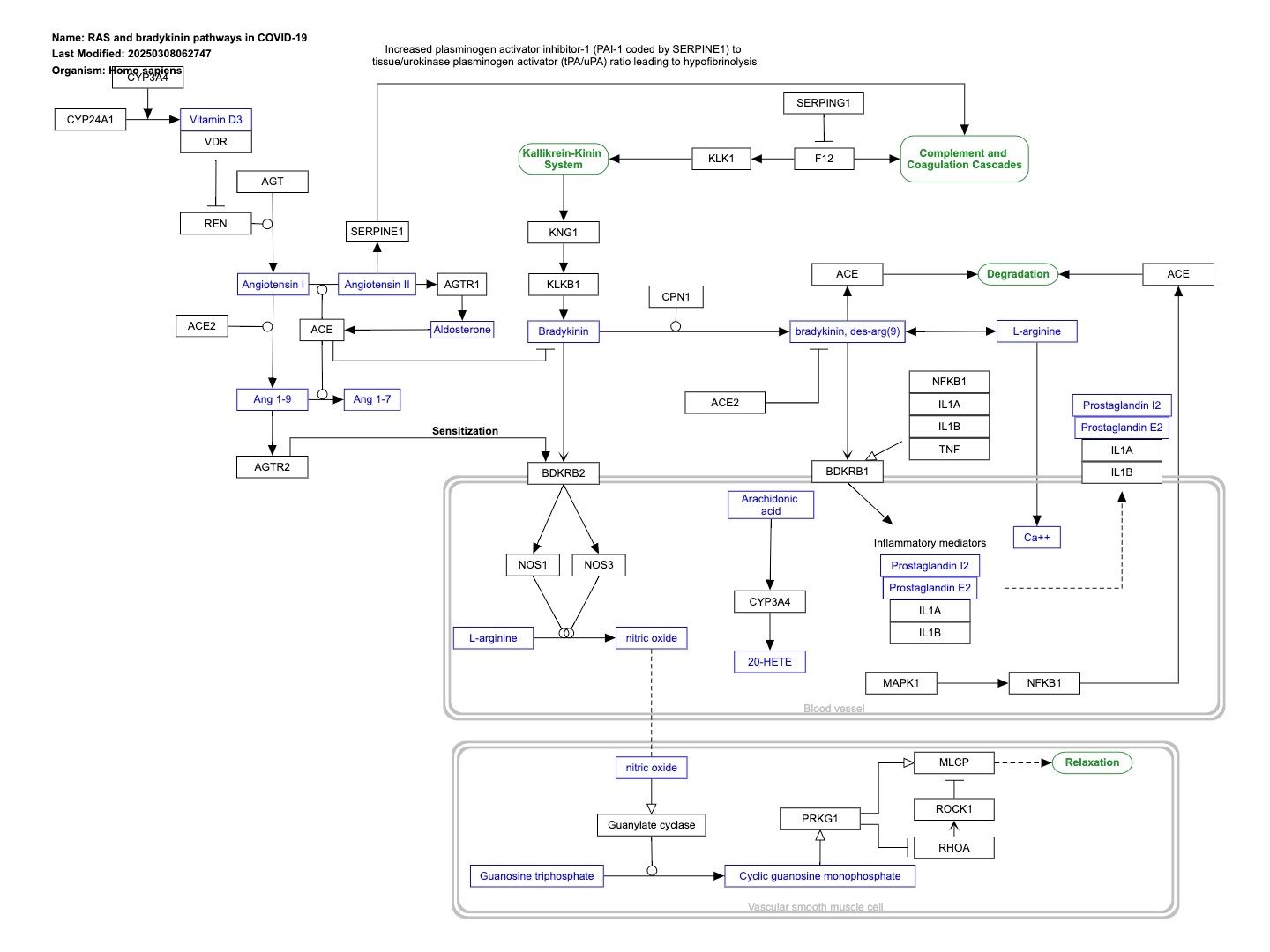
Interactive Pathways chart: https://pathway-viewer.toolforge.org/embed/WP4969
This pathway describes imbalances in RAAS and Bradykinin pathways in COVID-19. The expression of several genes in these pathways is affected in by SARS-CoV-2: * SERPING1 is downregulated, which cancels the suppression of F12 of the intrinsic coagulation cascade, resulting in the production of bradykinin from kallikrein and KNG. * ACE is downregulated, which increases bradykinin levels. * ACE2 is upregulated, ACE is downregulated, which causes an increase in Angiotensin 1-9 and sensitization of bradykinin receptors. * NFkappaB is suppressed by SARS-Cov-2, decreasing its binding to the ACE promoter and subsequent transcription. The result of a hyperactive bradykinin system is vasodilation to the point of vascular leakage and infiltration of inflammatory cells. The pathway is based on Figure 2A from Garvin et al..
Bradykinin, Cytokines and Inflammatory Hyperalgesia
Bradykinin (BK) and related peptides are produced at sites of tissue injury and inflammation and have an important role in mediating the responses of the tissues to damage and associated pain. BK causes pain by direct stimulation of nociceptors and by sensitising sensory fibres to previously non-noxious stimuli (hyperalgesia). BK exerts its biological activities by stimulating two receptor subtypes, B1 and B2, with the latter localised to sensory neurones. The B2 receptor is believed to mediate many of the effects attributed to kinins although the B1 receptor has now been shown to have an important role in hyperalgesia, notably when the hyperalgesia is secondary to an earlier inflammatory insult.
Pro-inflammatory cytokines, e.g. interleukin-1β (IL-1β) and tumour necrosis factor α (TNFα), are potent hyperalgesic agents that constitute a link between cellular injury and the development and resolution of local and systemic manifestations of inflammation. The release of TNFα by macrophages can be induced by BK and, in a model of mechanical hyperalgesia, both BK (the preferred agonist of the B2 receptor) and its metabolite, the B1 receptor agonist des-Arg9 BK, each initiates a cascade of cytokine release which mediates hyperalgesic responses to inflammatory agents. The cytokine cascade begins with TNFα, which stimulates production of IL-1β, IL-6, IL-8, prostaglandins (PGs) and sympathomimetics.
In the above model, B1 and B2 agonists act synergistically to cause cytokine-mediated hyperalgesia, and B1 and B2 antagonists proved to be highly effective anti-hyperalgesic drugs, especially when given together. A striking synergy between B1 and B2 receptor agonists, with each other and with IL-1β, was also described in a recent study in which B1 and B2 agonists, in the presence of IL-1β, shifted the repertoire of BK receptor subtypes from B2 to B1. Since, in addition to BK-induced IL-1β, IL-1β can induce B1 receptor-mediated hyperalgesia in models of mechanical and thermal hyperalgesia, there appear to be roles for both B1 and B2 receptors in a complex network of hyperalgesic mediators in which BK and related peptides induce cytokines and vice versa.
Another group of endogenous mediators, which includes the cytokines IL-4 and IL-1O and also lipocortin-l (LC-I), inhibit the production/action of TNFα, IL-Iβ, IL-6 and IL-8, and are now reported to have anti-hyperalgesic properties. The manipulation of the relative balance of these various proteins may permit the development of novel treatments of inflammatory hyperalgesia. In the meantime, the search for anti-hyperalgesic/analgesic drugs with novel mechanisms of action includes research on antagonists of BK B2 and, especially, B1 receptors (and possibly the combination of B1 and B2 receptor antagonists) and on the (orally active) tripeptide inhibitors of IL-Iβ-mediated hyperalgesia that have been described.
Clots, kinins and coronaries
Bradykinin is the major effector for the plasma kinin system and is released from high molecular weight kininogen following a complex interaction between Factor XII, Factor XI and prekallikrein on the surface of endothelial cells (Fig. 1) [10,11]. An alternative pathway exists in tissues whereby cleavage of low molecular weight kininogen by tissue kallikreins yields the decapeptide, Lys-bradykinin [12,13]. Subsequent removal of the N-terminal lysine of Lys-bradykinin by aminopeptidases generates bradykinin (Figs. 1 and 2) [12,13].
Mouse Models of Human Cardiovascular Disease
Bradykinin
Bradykinin is a product of the action of kallikrein on kininogen, and it is able to induce vasodilation and natriuresis by releasing NO and prostaglandins. Most cardiovascular and renal actions are mediated by activation of the bradykinin B2 receptors.
Pain Mechanisms and Management in the Rheumatic Diseases
Bradykinin
Bradykinin is an important mediator of inflammatory pain, causing nociceptor activation and sensitization via B2 receptors [39]. The abundant metabolite of bradykinin, des Arg9 bradykinin (kallidin), activates B1 receptors, which occur in low abundance, in the periphery and CNS [40–42].
B2 receptors undergo desensitization following prolonged kinin exposure, whereas B1 receptors do not desensitize rapidly and are dramatically upregulated in many tissues following injury [43–46], exposure to interleukin-1 beta (IL-1β), or the neurotrophin glial-derived neurotrophic factor (GDNF) [44,47]. Kinins cause a cascade of secondary changes, including prostanoid and nitric oxide production, phosphorylation of signaling proteins such as protein kinase-C, and the sensitization of sensory transducers, such as the transient receptor potential (TRP) V-1 receptor [48]. These events are linked with heat and mechanical hyperalgesia [49,50]. In keeping with this, B2 (eg, Icatibant, bradyzide) and the B1 antagonist (des Arg10 HOE-140; SSR240612) produce robust antihyperalgesic effects in models of nerve injury-induced pain [51–54]. Importantly, intra-articular administration of Icatibant (HOE 140) in OA patients was shown to reduce pain intensity at rest and during activity [55]. Other bradykinin antagonists in development include Amgen's AMG379 and Sanofi-Aventis SSR240612, both antagonists at the B1 receptor.
The Pathophysiology of Cardiac Hypertrophy and Heart Failure
Bradykinin
Bradykinin (BK) is a circulating peptide derived from high-molecular-weight kininogen. It acts primarily on endothelial cells in the peripheral and coronary vasculature. There are two subtypes of BK receptor, bradykinin receptor 1 (B1R) and bradykinin receptor 2 (B2R). Both receptors are in the G-protein-coupled receptor (GPCR) family, but mediate different actions. B1R is an inducible receptor that is expressed as a result of tissue injury. B1R signaling induces activation of calcium-dependent iNOS. B1R signaling promotes local tissue inflammation by recruiting neutrophils, dilating capillaries, and constricting venous outflow. Endothelial cells throughout the vascular system constitutively express B2R. B2R signaling causes increased intracellular calcium, causing activation of eNOS.283 Vasodilation occurs by release of NO to surrounding vascular smooth muscle cells (VSMC), where it induces formation of cGMP, a potent VSMC relaxant.284 B2R signaling is also believed to result in negative transcriptional regulation of proliferation and growth in VSMC and cardiomyocytes. This action counters both hypertrophy and arterial wall thickening.285 Importantly, BK is metabolized by the angiotensin-converting enzyme. Treatment with ACE inhibitors acts to potentiate serum levels of BK, resulting in a systemic vasodilatory effect (in addition to the direct effect of blocking production of the vasoconstrictor ATII).
Pain
5.30.2.4 Other Mediators: Bradykinin
Bradykinin is a nonapeptide generated from high-molecular-weight kininogen through cleavage by kallikrein under inflammatory conditions in plasma. It is also released from mast cells during asthma attacks and within the gut as a gastrointestinal vasodilator. Bradykinin is rapidly generated after tissue injury and seems to modulate most of the events observed during the inflammatory process including vasodilatation, increase of vascular permeability, plasma extravasation, and cell migration (reviewed in Calixto, J. B. et al., 2000). It is also one of the most potent endogenous proalgesic mediators (Ferreira, S. H. et al., 1993). It directly activates bradykinin receptors on primary afferent neurons or sensitizes nociceptors indirectly through release of prostaglandins, nitric oxide, neurokinins, calcitonin gene-related peptide, cytokines, and histamine (Figure 1), or via alterations of vanilloid receptor channel gating (Chuang, H. H. et al., 2001). In fact, lipopolysaccharide- or carrageenan-induced inflammation triggers the TNF-α-driven cytokine cascade via the release of bradykinin (references in Cunha, F. Q. and Ferreira, S. H., 2003). Bradykinin is released at the beginning of the inflammation because delayed treatment with bradykinin receptor antagonists 2 h after injection of lipopolysaccharide or carrageenan has no effect on hyperalgesia or inflammation.
-
New Therapeutic Strategies for Brain Edema and Cell Injury
2 Bradykinin is a mediator of edema formation
Bradykinin is a well-known mediator of the brain edema formation and BBB disruption (Gröger et al., 2005; Plesnila et al., 2001; Schulz et al., 2000). However, its role in BSCB permeability is still not clear (Bannister et al., 2014; Ma et al., 2019; Mandadi et al., 2016; Pan, Kastin, Gera, & Stewart, 2001; Sharma, 2000; Wahl, Görlach, Hortobágyi, & Benyó, 1999; Xu et al., 2008; Yan-Feng, Gang, & Yan-Ting, 2008). Increased bradykinin content following brain injury has been demonstrated. Bradykinin has 2 receptors such B1 and B2. The detail mechanisms of B1 and B2 receptors in mediating the BBB permeability however, is still not well known (Görlach et al., 2001; Görlach, Benyó, & Wahl, 1998; Görlach & Wahl, 1996; Gröger et al., 2005; Plesnila et al., 2001; Schulz et al., 2000; Schürer, Temesvari, Wahl, Unterberg, & Baethmann, 1989; Unterberg, Wahl, & Baethmann, 1984; Wahl et al., 1996; Wahl, Young, Edvinsson, & Wagner, 1983; Whalley & Wahl, 1983).
Both bradykinin and its receptors are present in healthy human brain and altered in disease processes such as ischemic brain injury (Bannister et al., 2014; Xu et al., 2008; Yan-Feng et al., 2008). Increased bradykinin concentration in the cerebrospinal fluid (CSF) is well documented for traumat6ic brain injury, ischemic stroke and intracerebral hemorrhages (Gröger et al., 2005; Kunz et al., 2013; Ma et al., 2019; Plesnila et al., 2001; Schulz et al., 2000; Schürer et al., 1989;Unterberg et al., 1984; Wahl et al., 1983; Whalley & Wahl, 1983). Increased bradykinin concentration correlates well with the severity of brain edema (Kunz et al., 2013; Unterberg et al., 1984; Unterberg, Polk, Ellis, & Marmarou, 1990; Wahl et al., 1983; Whalley & Wahl, 1983). Brain edema following increased bradykinin concentrations in the brain is largely due to bradykinin-induced vasodilatation together with venous constriction (Unterberg et al., 1984; Wahl et al., 1983; Whalley & Wahl, 1983). Bradykinin also increases microvascular permeability to plasma proteins causing fluid retention and development of cerebral edema (Pan et al., 2001; Sharma, 2000; Xu et al., 2008; Yan-Feng et al., 2008).
Several in vitro studies suggest that blockade of B2 receptors decreases the permeability of isolated cerebral endothelial cells and increased the electrical resistance. (Liu, Xue, Liu, & Wang, 2008; Raslan et al., 2010; Schöller, Feiler, Anetsberger, Kim, & Plesnila, 2011; Trabold et al., 2010). This suggest that blockade of B2 receptors somehow strengthen the endothelial cell permeability (Baethmann et al., 1983, 1988; Wahl et al., 1993, 1988, 1990). There are reports that activation of bradykinin B2 receptors increases the size of the lesion in stroke and enhances BBB breakdown resulting in local neuroinflammation (Görlach et al., 1998, 2001; Görlach & Wahl, 1996; Kunz et al., 2013; Pan et al., 2001; Schürer et al., 1989; Sharma, 2000; Unterberg et al., 1984; Wahl et al., 1999, 1996, 1983; Whalley & Wahl, 1983). Upregulation of bradykinin B2 receptors is seen as early as 2 h after the onset of ischemic stroke that continue to be elevated up to 24 h after the primary insult (Dobrivojević, Špiranec, & Sinđić, 2015; Gauberti, Potzeha, Vivien, & Martinez de Lizarrondo, 2018; Smeda & Daneshtalab, 2017). Interestingly, role of bradykinin and its receptors in SCI are still unclear and require further investigation (see Pan et al., 2001; Sharma, 2000).
2.1 Bradykinin stimulates nitric oxide production
Bradykinin induced vasodilatation is largely caused by endothelial production of nitric oxide (NO) (Genaro, Stranieri, & Borda, 2000; Landmesser & Drexler, 2006). Nitric oxide (NO) is a free radical gas and when produced in vivo could also affect microvascular permeability disturbances and cell injury (Sharma, 1998, 1999; Sharma, Alm, & Westman, 1998; Sharma, Drieu, Alm, & Westman, 2000). Our previous studies show that SCI leads to upregulation of neuronal nitric oxide synthase (nNOS) in the traumatized cord and the adjacent rostral and caudal segments (Sharma et al., 1998; Sharma, Westman, Olsson, & Alm, 1996). Upregulation of nNOS is well known to induce NO production that is responsible for cell injury and microvascular permeability disturbances (Sharma, 1998; Sharma & Alm, 2004; Sharma, Alm, & Westman, 1998; Sharma, Nyberg, Westman, et al., 1998; Sharma et al., 1996). This is further substantiated by our findings that when nNOS antibodies were applied topically over the injured spinal cord, cell changes, edema formation and nNOS upregulation in the spinal cord were absent (Sharma et al., 1996; Sharma, Nyberg, Westman, et al., 1998).
In cell culture studies, bradykinin stimulated nitric oxide production by twofold within 30 min after its application. This NO stimulation by bradykinin is blocked by bradykinin B2 receptor antagonist HOE-140 (Sesti, Martino, Mazzulla, & Chimenti, 2005; Zhang, Nasjletti, Xu, & Hintze, 1998). This observation clearly suggests that bradykinin induced endothelial NO production is mediated by bradykinin B2 receptors.
2.2 Bradykinin B2 receptors in spinal cord injury
Bradykinin is implicated in spinal network, neuropathic pain, rhizotomy, inflammatory hyperalgesia and neuroinflammation in the spinal cord (Bannister et al., 2014; Ma et al., 2019; Mandadi et al., 2016; Pan et al., 2001; Sharma, 2000; Xu et al., 2008; Yan-Feng et al., 2008). Very few report deal with bradykinin in spinal cord injury (Pan et al., 2001; Sharma, 2000). There are reports of significant elevation of bradykinin after experimental spinal cord injury (see Pan et al., 2001). Also interaction of bradykinin with aquaporin4 in spinal cord ischemic injury to the cord was described. (Xu et al., 2008) We reported reduction in BSCB breakdown by B2 receptor antagonist HPE-140 in cord injury indicating involvement of bradykinin in SCI (see Sharma, 2000). However, detailed investigations on bradykinin involvement in SCI require further investigations. With view of bradykinin interacting with dynorphin and nitric oxide in the CNS it is important to understand the modulating roles of bradykinin in SCI that upregulated both nitric oxide and dynorphin after trauma (see Bannister et al., 2014; Sharma, Nyberg, Gordh, et al., 1998; Sharma, Nyberg, & Olsson, 1992; Sharma, Olsson, & Nyberg, 1995; Sharma et al., 1996; Stålberg et al., 1998; Winkler, Sharma, Stålberg, & Westman, 1998).
Peat on Bradykinin
Direct mentions are sparse:
"Bacterial endotoxin increases serotonin release from the intestine, and increases its synthesis in the brain (Nolan, et al., 2000) and liver (Bado, 1983). It also stimulates its release from platelets, and reduces the lungs' ability to destroy it. The formation of serotonin in the intestine is also stimulated by the lactate, propionate and butyrate that are formed by bacteria fermenting fiber and starch, but these bacteria also produce endotoxin. The inflammation-producing effects of lactate, serotonin, and endotoxin are overlapping, additive, and sometimes synergistic, along with histamine, nitric oxide, bradykinin, and the cytokines."
"Y. Kuraishi (2015) said that noradrenaline inhibits pain by inhibiting the release of substance P and glutamate (the excitatory amino acid), and that the suppression of cancer pain results in the inhibition of tumor growth and lung metastasis..., apparently by inhibiting the release of substances from cancer cells (e.g., ATP, endothelin-1, and bradykinin). Things that activate and enliven the patient, and that at the same time decrease pain, seem to be therapeutically appropriate."
A role for bradykinin signaling in chronic vulvar pain
Chronic vulvar pain is alarmingly common in women of reproductive age and is often accompanied by psychological distress, sexual dysfunction, and a significant reduction in quality of life. Localized provoked vulvodynia (LPV) is associated with intense vulvar pain concentrated in the vulvar vestibule (area surrounding vaginal opening). To date, the origins of vulvodynia are poorly understood, and treatment for LPV manages pain symptoms, but does not resolve the root causes of disease. Until recently, no definitive disease mechanisms had been identified; our work indicates LPV has inflammatory origins, although additional studies are needed to understand LPV pain. Bradykinin signaling is one of the most potent inducers of inflammatory pain and is a candidate contributor to LPV. We report that bradykinin receptors are expressed at elevated levels in LPV patient versus healthy control vestibular fibroblasts, and patient vestibular fibroblasts produce elevated levels of proinflammatory mediators with bradykinin stimulation. Inhibiting expression of one or both bradykinin receptors significantly reduces proinflammatory mediator production. Finally, we determined that bradykinin activates NFκB signaling (a major inflammatory pathway), while inhibition of NFκB successfully ablates this response. These data suggest that therapeutic agents targeting bradykinin sensing and/or NFκB may represent new, more specific options for LPV therapy.
Bradykinin in Pain & Inflammation + How to Decrease It
Positive Effects of Bradykinin
Although bradykinin may cause problems, it also serves an important purpose in the body.
Bradykinin protects your heart and your nerves from damage. In the heart, it widens vessels and lowers blood pressure, which prevents heart failure and damage caused by high blood pressure. When the spinal cord or other nerves are injured, bradykinin protects nerve cells and prevents them from dying [15, 16].
1) Heart Health and Blood Pressure
High blood pressure can lead to heart failure. Bradykinin widens blood vessels and decreases blood pressure, which can protect the heart and keep it functioning after congestive heart failure [17, 18].
Doctors often take advantage of the protective effects of bradykinin to help patients with heart conditions. Angiotensin-converting-enzyme inhibitors, or ACEIs, are often prescribed to prevent heart failure in people with high blood pressure. ACEIs stop bradykinin from being broken down, and the increased concentration of bradykinin decreases blood pressure [15, 19].
2) Insulin Sensitivity
Bradykinin makes fat and muscle cells more sensitive to insulin, possibly by increasing the activity of insulin receptors on these cells [20].
Insulin resistance, the condition in which cells are not sensitive enough to insulin, develops before and often predicts type 2 diabetes. Because bradykinin increases the insulin sensitivity of certain cells, it may reduce the risk of developing type 2 diabetes. In turn, drugs which increase bradykinin, such as ACEIs, may prevent type 2 diabetes from developing [21, 20].