The "essential" amino acids may be even more detrimental than EFA
-
@TexugoDoMel good to know so beans and muscle meat may be ok once in a while
-
@thyroidchor27 Yes, milk and cheese is also very high in methionine.
I think cycling is an option or, balancing it out. Getting half of the protein from muscle meat/milk and the other half from gelatin.
-
Just to add a little bit more...
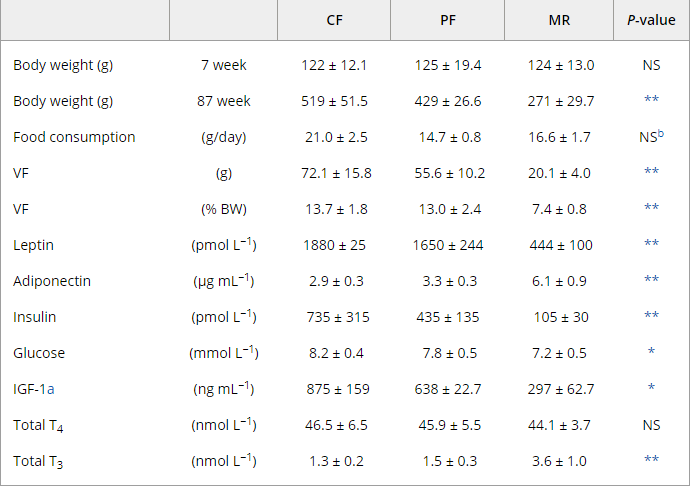
Look at total T3 from MR rats. Total T4 is the same as the others, which indicates a greater conversion of T4 to T3
-
@TexugoDoMel Thanks for sharing!
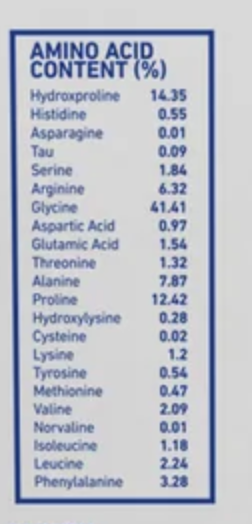
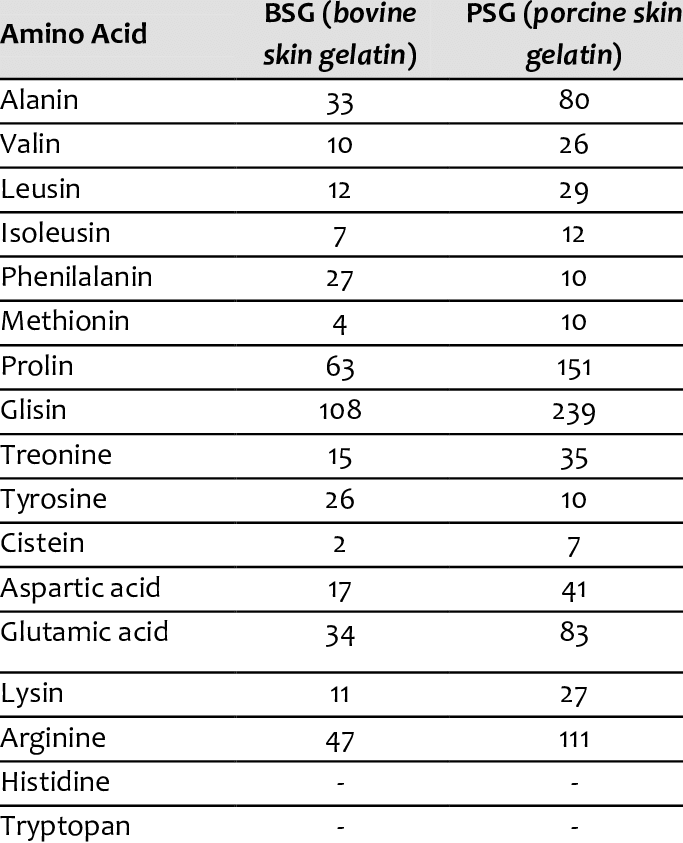
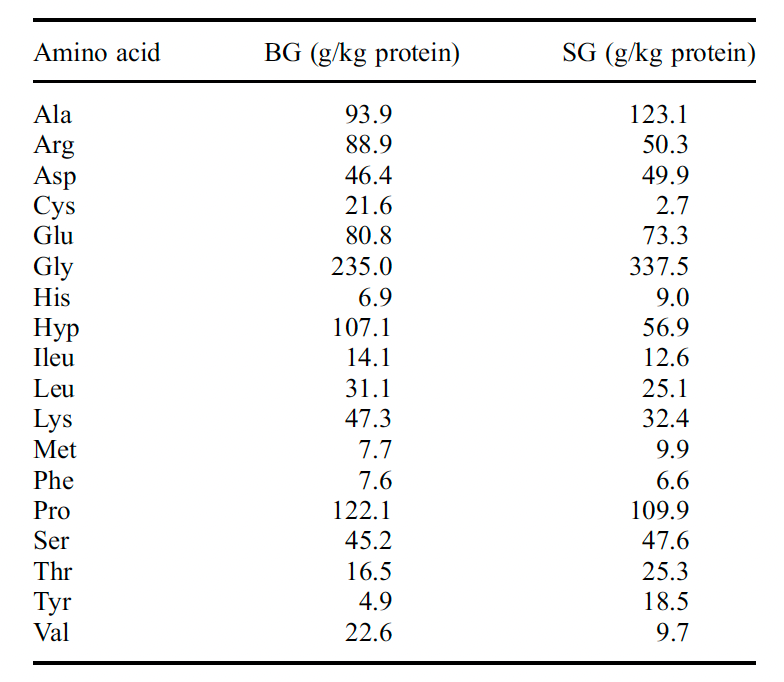
Do you have a reference for the picture?
-
@Kasper
" the increased DEE observed in mature (63-week-old) MR animals relative to PF and CF probably resulted from increased peripheral conversion of T4 to T3, since total T4 levels were equivalent in all groups."https://onlinelibrary.wiley.com/doi/full/10.1111/j.1474-9726.2006.00220.x
-
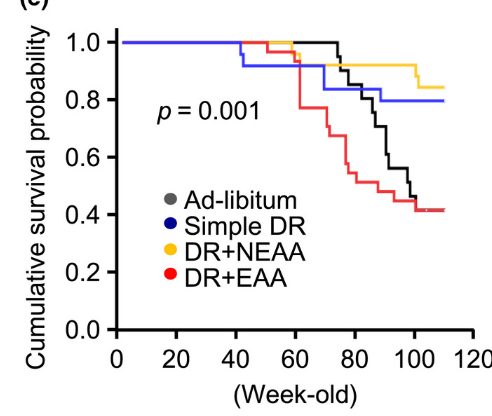
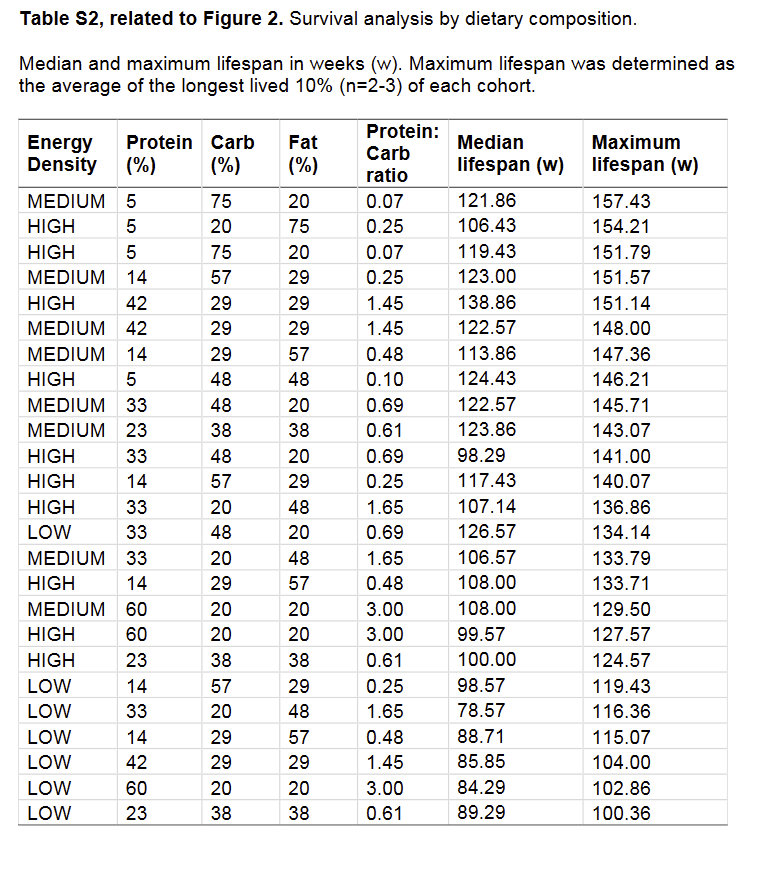
While the evidence for protein restriction with regards to life extension is very clear, there are some subtle points that have yet to be clarified. For example, in Solon-Biet et al. (2014) the group of mice with the highest median life expectancy was a group eating 42% protein, with a maximum life span only slightly smaller than the very-low-protein groups.
-
@Kvothe Interesting, any ideas about that?
Also all the low energy density diets seemed to be detrimental to lifespan.
-
@Kasper No, just that it's complex. Whether or how harmful protein is probably depends on a lot of factors that are not mentioned or measured in most studies. There might be a lot of things at play we don't see. For example, what explanation is there for the fact that animals eating a high energy, high protein diet with 42% protein, and 29% carbs and fat have a significantly larger median life span (138 vs 98 days) than those eating a high energy, high protein diet, with 33% protein, 48% carbs, and 20% fat?
-
ray said that in a healthy adult, only a few milligrams daily of the essential amino acids are necessary. If one is still growing, more is likely necessary, and if you're damaging tissue through weight training, compensatory measures should probably be taken.
-
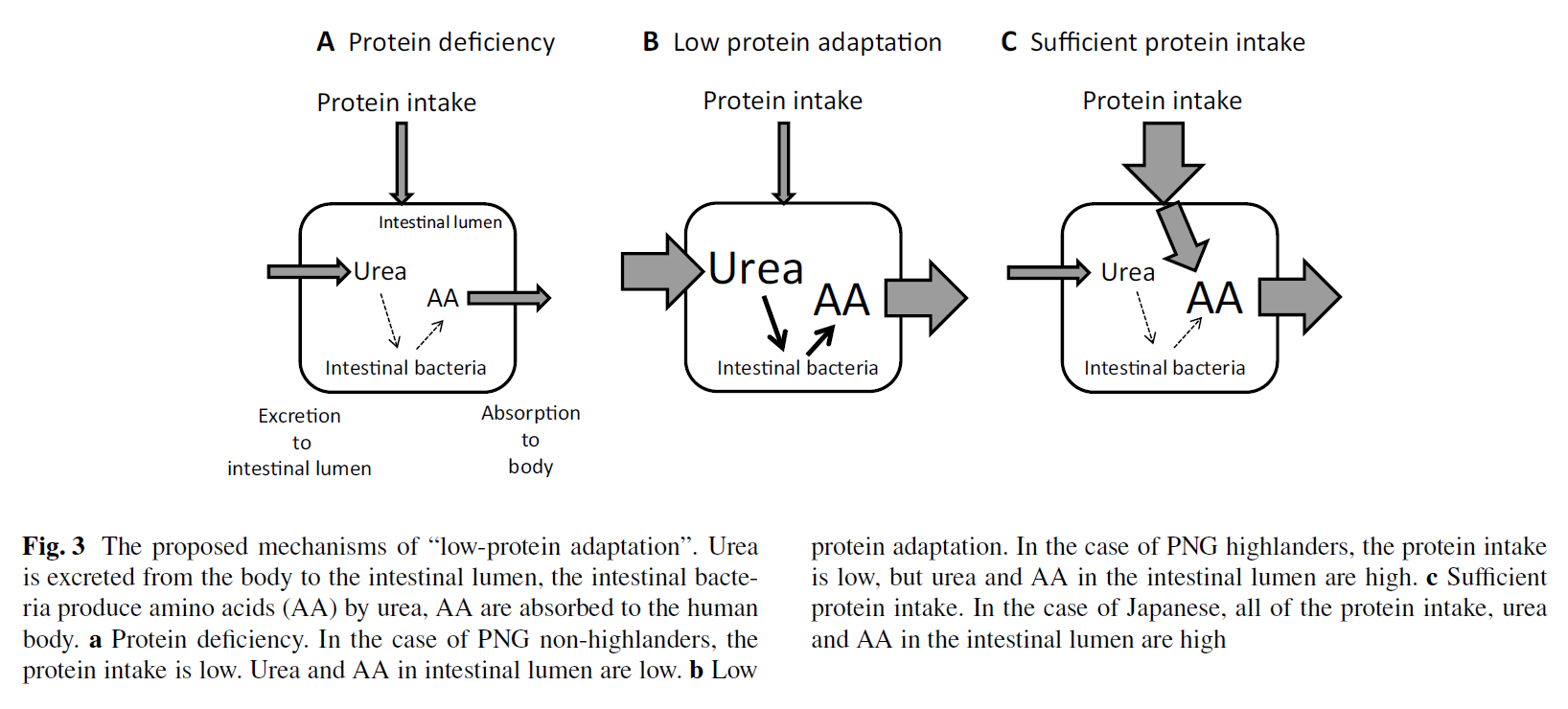
Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in CKD
"The rise in urea concentration in the intra-cellular and extra-cellular fluid compartments results in its heavy influx into the gastro-intestinal tract (7). Within the intestinal lumen urea is hydrolyzed by microbial urease forming large quantities of ammonia [CO(NH2)2 +H2O → CO2+2NH3] which is readily converted to ammonium hydroxide [NH3 + H2O → NH4OH] (8,9). Ammonium hydroxide, in turn, leads to a modest rise in the luminal fluid's pH, causes mucosal irritation and promotes enterocolitis [10,11]. Based on these observations we hypothesize that ammonium hydroxide generated from hydrolysis of urea by microbial urease in the intestinal lumen of uremic patients may contribute to the epithelial barrier dysfunction and erosion of the TJ protein constituents."
"The present study revealed that at clinically-relevant concentrations, urea significantly lowered the TER [Transepithelial Electrical Resistance] in the tight junction-forming polarized human colonic epithelial cell monolayer in vitro. The effect of urea was dramatically amplified by urease which was used to simulate the effect of urease-possessing microbial species in the colon. These observations illustrate the potential contribution of influx of urea into the intestinal tract in the pathogenesis of the uremia-induced intestinal barrier dysfunction in the uremic patients. The observed fall in the TER was accompanied by and was largely due to the urea-concentration-dependent depletion of occludin, claudin 1, and ZO1 which are the key protein constituents of the epithelial TJ. The erosive effect of urea on the measured TJ proteins was greatly intensified in the presence of urease leading to near complete depletion of ZO1 and occludin and severe reduction in claudin 1 abundance."
"As noted above, accumulation of urea in the intra- and extra-cellular fluid compartments in patients and animals with advanced CKD results in its heavy influx into the gastro-intestinal tract via passive diffusion and incorporation in the glandular secretions (7–9). As noted earlier within the intestinal lumen urea is hydrolyzed spontaneously and by microbial urease forming large quantities of ammonia [8,9]. Ammonia is, in turn, converted to ammonium hydroxide which is a caustic base capable of causing cytotoxicity and tissue damage. Recent studies conducted by our group have shown marked changes in the composition of microbial flora in humans and animals with advanced CKD [21]. Five of the 10 microbial families which were most abundant in the CKD patients possessed functional urease [2,21]. Thus massive influx of urea into the gastrointestinal tract together with the dominance of urease-possessing bacteria in the uremic individuals, work in concert to promote formation of ammonia and ammonium hydroxide in the intestinal tract [8,9]. This can account for the dramatic impairment of the barrier function and destruction of TJ apparatus observed in the present in vitro experiments designed to simulate the effect of uremia in vivo."