On choline
-
"[..]it is well documented that the mouse model of chronic choline deficiency differs from rats because most mouse strains do not develop liver cirrhosis and hemorrhagic necrosis that is typical of chronic choline deficiency in rats (DeCarmago et al., 1985). It is also generally recognized that susceptibility to develop fatty liver in choline deficiency is age-dependent. The majority of studies examining choline deficiency began dietary modulation in weanling animals, which are highly susceptible to choline deficiency (Rogers et al., 1987). Susceptibility to choline deficiency declines rapidly, and young adults (10–12 weeks of age) are unlikely to develop features of fully expressed choline deficiency. Secondly, dietary fat composition also contributes to the development of fatty liver in choline deficiency. Specifically, the fat composition of choline deficient diets is often augmented to at least 20%, whereas standard laboratory chows contain about 5% fat. Additionally, variations in fatty liver development are observed when the fat is derived from animal or plant origins (Rogers et al., 1987; DeCarmargo et al., 1985)."
"[..]there are marked species differences in susceptibility to choline deficiency, with rats and mice being far more susceptible than other species including humans (Zeisel and Blusztajn, 1994). These differences are attributed to quantitative differences in the enzyme kinetics controlling choline metabolism. Rats and mice rapidly metabolize choline to betaine in the liver and it is likely that choline oxidase activity determines choline requirements and controls species sensitivity to choline deficiency (Sidransky and Farber, 1960). For example, choline oxidase activity is much lower in primates than rodents and primates are less sensitive to choline deficiency (Hoffbauer and Zaki, 1965). Humans have the lowest choline oxidase activity of all species and are generally refractory to choline deficiency, with evidence of choline deficiency observed only after prolonged fasting, significantly depressed liver function or deficient parenteral feeding (Zeisel and Blusztajn, 1994)."
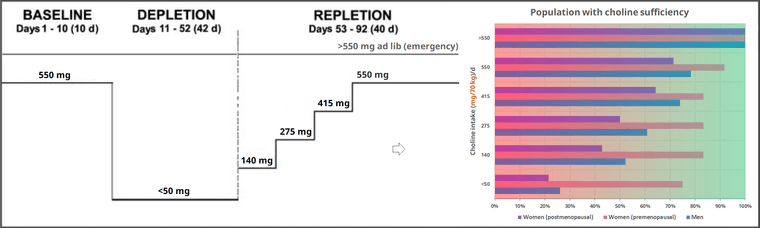
Sex and menopausal status influence human dietary requirements for the nutrient choline
Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione (on immature mice)
"The pathogenesis and treatment of nonalcoholic steatohepatitis (NASH) are not well established. Feeding a diet deficient in both methionine and choline (MCD) is one of the most common models of NASH, which is characterized by steatosis, mitochondrial dysfunction, hepatocellular injury, oxidative stress, inflammation, and fibrosis. However, the individual contribution of the lack of methionine and choline in liver steatosis, advanced pathology and impact on mitochondrial S-adenosyl-L-methionine (SAM) and glutathione (GSH), known regulators of disease progression, has not been specifically addressed. Here, we examined the regulation of mitochondrial SAM and GSH and signs of disease in mice fed a MCD, methionine-deficient (MD), or choline-deficient (CD) diet. The MD diet reproduced most of the deleterious effects of MCD feeding, including weight loss, hepatocellular injury, oxidative stress, inflammation, and fibrosis, whereas CD feeding was mainly responsible for steatosis, characterized by triglycerides and free fatty acids accumulation."
[..]the decrease in membrane fluidity induced by the lack of methionine is accompanied by a lower PC/PE ratio, which is also considered an important modulator of membrane fluidity. Indeed, previous findings in PE methyltransferase knock-out mice fed a CD diet resulted in steatohepatitis due to low plasma membrane PC/PE ratio, which contributed to disrupted membrane integrity and decreased membrane fluidity (31). The observed outcome is intriguing as, unlike MCD diet, the MD diet contains an adequate choline level, which would be expected to drive the CDP-choline branch of the Kennedy pathway to synthesize PC de novo (11, 32), although the PC synthesis from PE methylation would be predicted to be impaired due to the limitation of SAM. To account for this unexpected finding (decreased PC/PE ratio despite choline in the MD group), we observed that methionine deficiency increased hepatic ceramide levels in agreement with previous observations in PC12 cells (35) and in mice fed the MCD diet (37). Although we did not determine whether the lack of methionine stimulated de novo ceramide synthesis from palmitoyl-CoA and serine, we did observe the activation of ASMase but not NSMase. The mechanism, however, underlying this observation is currently unknown and deserves further investigation. Moreover, based on previous observations in neuroblastoma cells exposed to C2-ceramide, the increase in ceramide levels induced by MD or MCD would be expected to inhibit the CDP-choline pathway and hence the synthesis of PC (34). The alternative synthesis of PC from PE by PE methyltransferases is anticipated to be impaired without methionine because of reduced SAM availability. Thus, the impact of methionine deficiency on mitochondrial GSH depletion is mediated by increasing ceramide levels and subsequent impaired PC synthesis (from either the CDP-choline pathway and PE methylation) resulting in lower PC/PE ratio, which determines reduced mitochondrial membrane fluidity and impaired transport of cytosolic GSH into mitochondria."
"Having observed the depletion of mitochondrial GSH in the MCD model (which is reproduced by MD diet feeding), we next addressed the effect of GSH precursors in the course of disease and replenishment of mitochondrial GSH. Our findings indicate that not all GSH prodrugs are equal. Although CysNAc, a GSH precursor, reverses the moderate depletion of hepatic GSH, it fails to restore the mitochondrial pool of GSH following MCD or MD feeding. The newly synthesized GSH from CysNAc in the cytosol is not effectively transported to mitochondria because of the block imposed by the loss of membrane fluidity associated with a decreased PC/PE ratio, consistent with previous observations in alcohol-fed rats (36). In a total enteral nutrition, the NASH model induced by overfeeding a diet enriched in polyunsaturated fat, CysNAc attenuated the progression of the liver pathology, which was associated with increased total hepatic GSH (39). However, the effect of CysNAc on the specific pool of mitochondrial GSH was not examined, nor was the impact of overfeeding on mitochondrial membrane composition and dynamics. In contrast to CysNAc, we report for the first time that GSH-EE [ethyl ester] attenuates the deleterious effects of feeding the MCD diet in terms of hepatocellular damage, fibrosis, and inflammation, and these effects are due to its ability to replenish mitochondrial GSH. Unlike CysNAc, GSH-EE is permeable to mitochondria and has been shown to boost GSH stores directly in conditions of impaired transport imposed by perturbed membrane fluidity (36)."
-
Glycerophosphocholine (GPC) is the primary form of choline that occurs in milk. It's purposed as a cognitive enhancer, but the molecule yields different products after metabolism, with a distribution beyond the brain.
The dose used below, extrapolated to humans, would be something like 1 g/70 kg, which isn't too crazy:
"In supplements, choline has most often been used by adults in doses of 1-3 grams by mouth daily for up to 4 months."
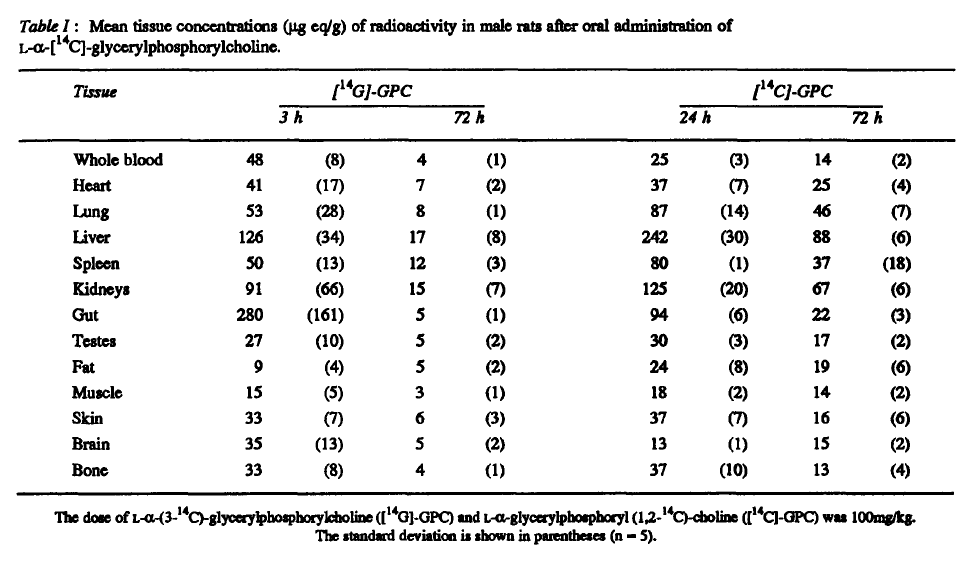
"aGPC was labelled with [14C]-glycerol ([14G]-GPC) or [14C]-choline ([14C]-GPC)."
"Both labelled compounds gave a wide distribution of radioactivity, particularly concentrated in the liver, kidney, lung and spleen compared to blood."
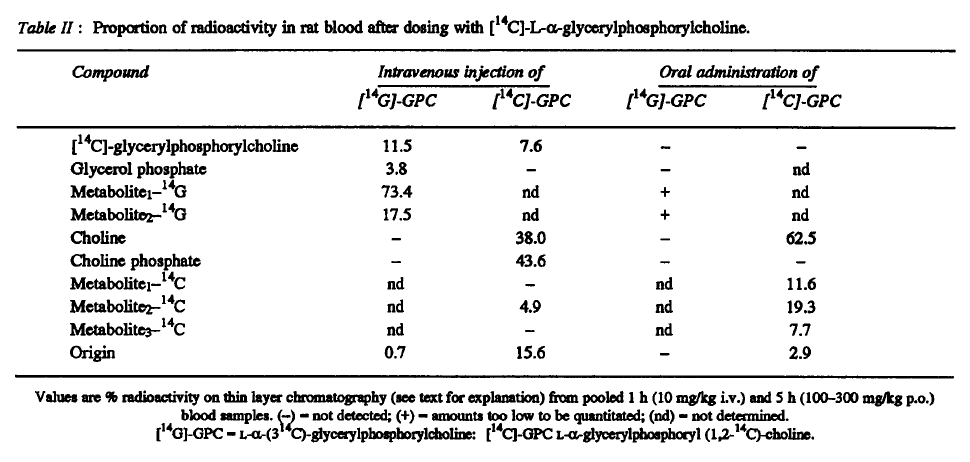
"After oral administration, the main circulating metabolite was choline (after [14C]-GPC); intact a-GPC was not present after either labelled compound although there were the same unknown metabolites as after i.v. injection."
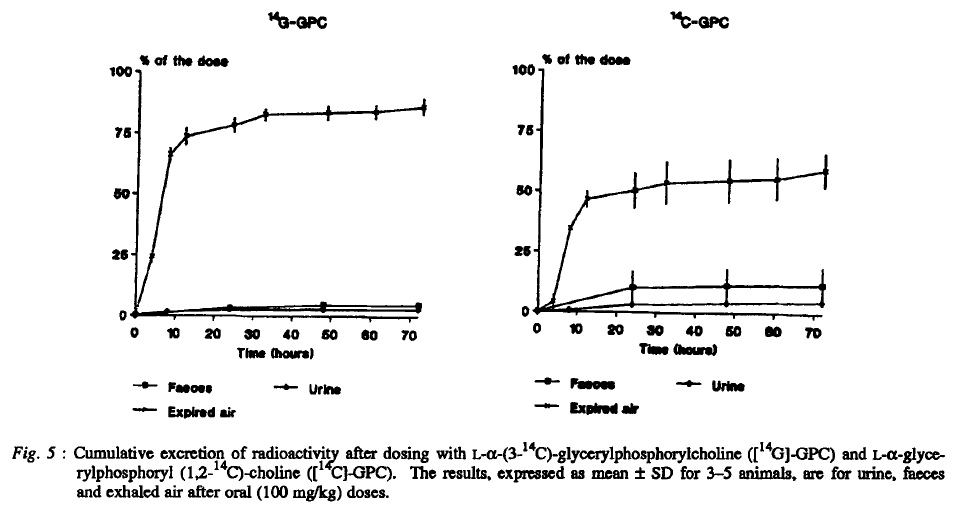
"[..]choline and/or its anabolites enter the brain and is reused for biosynthesis of phospholipids. This was confirmed by chromatography of the phosphatidylcholine fraction of the brain homogenates after hydrolysis with phospholipase C. As in blood, this hydrolysis resulted in formation of choline phosphate."
"By far, the largest part of the administered radioactivity was exhaled as 14CO2, in accordance with established catabolic pathways of glycerol (i.e. glycolysis) and choline degradation."
-
https://lowtoxinforum.com/threads/incomprehensive-ble-notes-on-choline.23228/
- A Cancer Therapy By Max Gerson - Selected Parts
[..]effects of choline and methionine [in protecting the liver] can be reversed by excess fat supplements.
With the tremendous amount of experimental work done on lipotropic agents, and their effectiveness in dietary fatty liver in animals, it is only natural that clinicians should turn to these substances in the treatment of fatty liver; however, the only type of fatty liver that choline (the most important of the lipotropic substances) can cure is the one due to a choline deficiency. It is likely that at least some of the fatty livers in man are due to choline deficiency, but in fatty livers of prolonged infection or those due to toxins, no deficiency of choline in the diet can be postulated, and therefore, no beneficial effect from choline can be expected.
Mitchell A. Spellberg, Diseases of the Liver, p. 309.