Zinc supplementation
-
"[..]the chemical form of zinc (salt or chelate), the pharmaceutical formulation (presence of excipients), and the division of the administered dose are other factors that modify the oral bioavailability of zinc [18]. Considering the pharmaceutical formulation, Neve et al [18] noted a decrease in the oral bioavailability of zinc when zinc gluconate was associated with starch, lactose, hydrated silica, and magnesium stearate (excipients present in Rubozinc), compared with zinc gluconate without excipients."
"In the case of our results, the significantly higher oral bioavailability of zinc when administered as bis-glycinate compared with gluconate is not surprising, given that bis- glycinate enhances the oral bioavailability of others ions, particularly iron."
"The higher absorption of zinc when chelated with bis-glycinate may well be explained by its chemical structure [27].
- Firstly, each glycinate molecule contains two functional groups that are capable of entering into covalent and coordinate covalent bonds with zinc.
- Secondly, each one can create a ring structure with the zinc ion and form a sterically and energetically stable structure.
- Thirdly, zinc bis-glycinate has a low molecular weight (< 1000 daltons) and is able to cross cell membranes.
- Lastly, the bis-glycinate chelate is not affected by pH changes from 2 to 6 [28].
Consequently, bis-glycinate may protect a significant fraction of the zinc from interactions with lumenal gastro-intestinal components, dietary components, and drugs [29] whereas in the current salts, the zinc may not be protected from such interactions."
Zinc Absorption Adapts to Zinc Supplementation in Postmenopausal Women
"To help establish common baseline conditions for all subjects, this double-blind study began with a 12-d pre-treatment period during which all subjects consumed the basal diet plus 6.0 mg Zn (as Zn gluconate) and 1.0 mg Cu (as Cu sulfate) daily. For the remaining 156 days, subjects continued the basal diets but with supplemental Cu reduced to 0.5 mg/d, and with random assignment of 9, 27 or 42 mg/d supplemental Zn, for total average Zn intakes of 14 (n = 6), 32 (n = 3), or 47 mg/d (n = 3). Both Zn and Cu supplements were divided into three equal portions, mixed with juice, and given with each of the three main meals, daily."
"Initially (wk 0) the women consuming 14, 32, and 47 mg Zn/d absorbed 4.6, 8.7, and 10.3 mg Zn (pooled SD = 1.3), respectively, suggesting a partial saturation of Zn absorption kinetics when Zn intakes met or exceeded 32 mg/d. However, with time, Zn absorption by those ingesting 32 and 47 mg Zn/d declined so that differences in total Zn absorbed were not significant at 8 wk (5.4, 5.8, 6.4 mg/d, respectively) and were negligible by 16 wk (5.0, 5.0, 5.1 mg/d)."
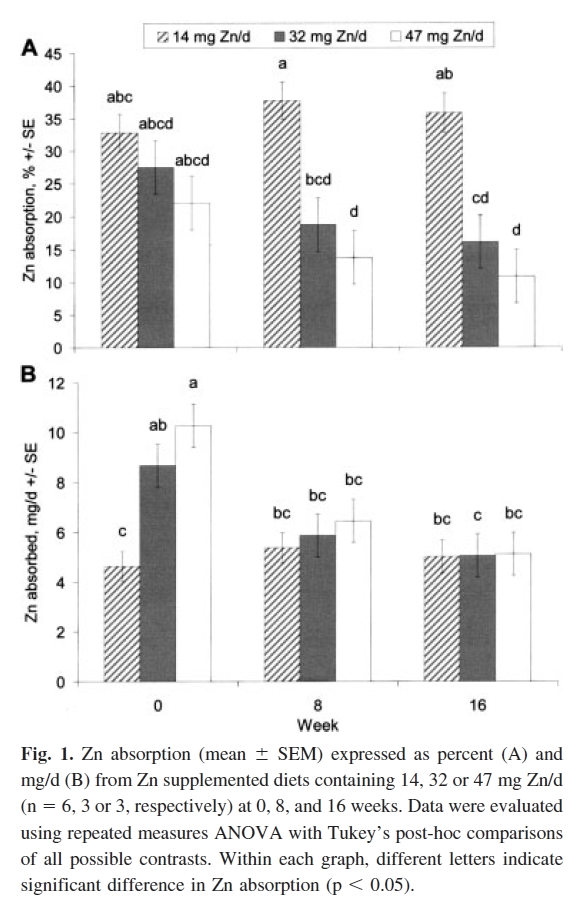
"The final absolute Zn absorption at 16 wk was [..] strikingly constant for all subjects, consistent with the overall finding that complete adaptation occurred (Fig. 1). Given the unusually extended period of highly controlled feeding conditions, and the precision of the whole body counting method, these significant results provide valuable information on human Zn homeostasis despite the small number of subjects."
"The complete absorptive adaptation observed in this study may be limited to adults with adequate Zn status who consume relatively low-phytate diets."
-
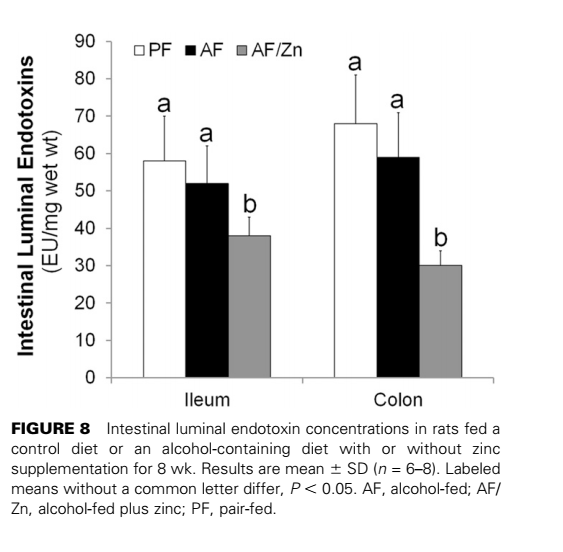
Do you remember that study we once discussed showing that SFA reduce the amount of endotoxin in the small intestine and colon? The same author bascially looked at the same parameters with zinc - turns out is lowers the total amount of endotoxin in the intestinal lumen to a similar extent.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4656905/
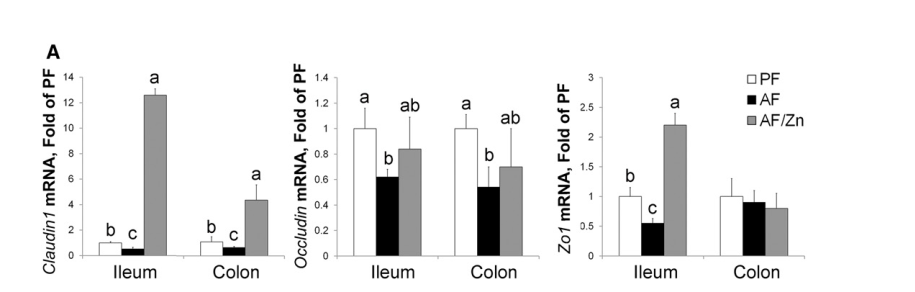
Zinc has a remarkable effect on Claudin1 expression.

-
https://www.bioenergetic.life/clips/839bf?t=4951&c=121
when asked about zinc, in this interview Dr. Peat said:
"Gluconate is okay, but I don't think doses above 10 milligrams per day are safe." -
Trace Element Speciation in Food
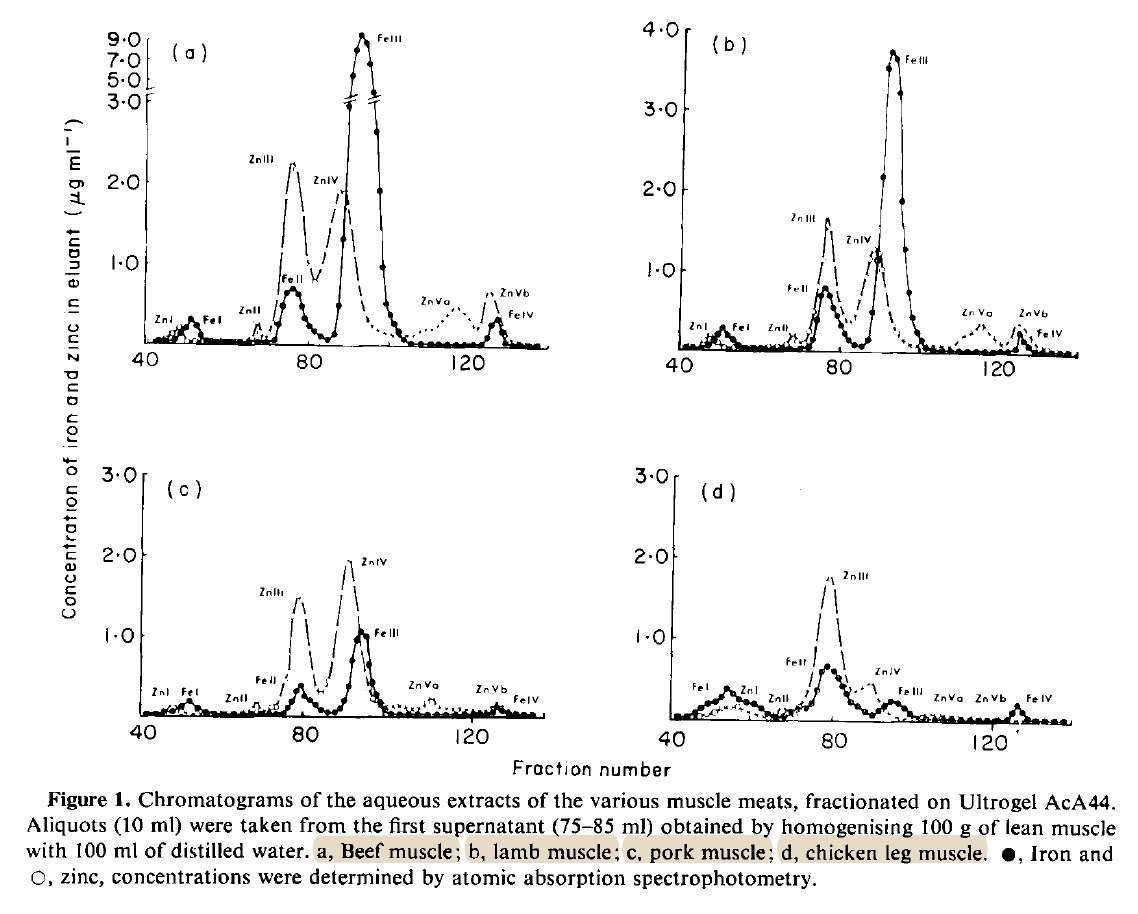
↳ [54] Iron and Zinc Compounds in the Muscle Meats of Beef, Lamb, Pork and Chicken"Figure 1 shows that the soluble zinc in all meats is distributed between five main components.
-
The first zinc peak corresponds to the exclusion volume of the column (molecular weight approximately 130 000) and therefore represents high molecular weight zinc. The zinc in this peak is possibly associated with a-macroglobulin which has a molecular weight of 820 000 and is known to account for one-third of all plasma zinc.[30]
-
Zinc peak II eluted in the same volume as the reference protein, transferrin, at a molecular weight of 76 000. Transferrin is known to bind a small amount of plasma zinc.[30]
-
The third zinc peak is very large and eluted at a molecular weight of approximately 65 000. Some, if not all, of the zinc in this peak is probably bound to albumin (molecular weight 67 000). Albumin is one of the sarcoplasmic proteins of meat[31] and is the predominant form of zinc in plasma.[30]
-
Zinc peak IV is also large and eluted at a molecular weight of approximately 35 000. This peak probably consists of the zinc metallo-enzyme carbonic anhydrase which has the same molecular weight, is a major soluble form of zinc in liver,[32] and accounts for nearly all the zinc found in erythrocytes.[30]
-
The fifth zinc peak eluted in a volume corresponding to low molecular weight compounds (< 12 000). The fractions from zinc peak Vb reacted with ninhydrin which suggests that this peak consists of zinc bound to amino acids.[19] Fractions from peak Va showed no reaction with ninhydrin and therefore probably consist of zinc bound to small peptides."
-