Vitamin C (IV route) doubles survival time in advanced pancreatic cancer patients
-
@Mossy in my opinion it's just better to take them separately. dha can irreversibly undergo hydrolysis in plain water and depending on how fast/slow digestion is maybe it starts to break down before it reaches target tissues. I think it makes more sense to let ascorbic acid and methylene blue meet in blood around target tissues and turn into dehyroascorbate and leucomethylene blue there so the dehydroascorbate can be taken up by tissues more immediately.
at most I suggest just mixing them right away and drinking, and not waiting for the solution to turn from blue to clear. -
@sneedful said in Vitamin C (IV route) doubles survival time in advanced pancreatic cancer patients:
@Mossy in my opinion it's just better to take them separately. dha can irreversibly undergo hydrolysis in plain water and depending on how fast/slow digestion is maybe it starts to break down before it reaches target tissues. I think it makes more sense to let ascorbic acid and methylene blue meet in blood around target tissues and turn into dehyroascorbate and leucomethylene blue there so the dehydroascorbate can be taken up by tissues more immediately.
at most I suggest just mixing them right away and drinking, and not waiting for the solution to turn from blue to clear.Thank you. You've answered a question I had asked on RPF, a few years back. I was curious if you could simply take both and let the reaction take place internally. You don't mention K2, but I'll assume the same.
-
@Mossy same deal with k2 . I assume you mean mk4. k2 mk4 best absorbs with lots of fat anyways and im not sure eating a bunch of fat with methylene blue seems like an appetizing combination
-
@sneedful said in Vitamin C (IV route) doubles survival time in advanced pancreatic cancer patients:
@Mossy same deal with k2 . I assume you mean mk4. k2 mk4 best absorbs with lots of fat anyways and im not sure eating a bunch of fat with methylene blue seems like an appetizing combination
Yes, K2, mk4. Thank you.
-
@Mossy oh wait I said methylene blue fat thing that doesn't make sense cause u would be taking k2 mk4 instead. nvm. u get what I mean.
-
btw @haidut afaik dhaa works via cancer cell death known as autoschizis. in your article you say dhaa corrects the reductive stress and makes cancer cells respire back to normal. maybe you’re also right too but i feel as if by leaving the cell killing part of it out it is missing part of the story. mayhaps dhaa both kills the deranged cells that are too deranged, and converts others back to normal respiration
-
@haidut said in Vitamin C (IV route) doubles survival time in advanced pancreatic cancer patients:
The bioenergetic theory states that cancer cells uptake more glucose from the blood because they are functionally deficient in glucose. Namely, the glucose that enters cancer cells gets “wasted” by conversion into lactate, due to the cancer cells’ highly reductive state (e.g. low mitochondrial NAD+/NADH ratio) instead of being combusted through OXPHOS. So, the solution would be to provide more glucose, not less. Be that as it may, the Apatone approach is to use a molecule (vitamin C) that uses the same uptake/transport mechanisms as glucose, which results in its preferential accumulation inside cancer cells, and once inside the cancer cell this oxidized form of vitamin C (DHAA) corrects the reductive excess of cancer cells, and after some time they start to respire normally. In other words, Apatone works by converting “cancer” cells back into “normal” cells by gradually changing the redox state of such cells from high reduced to highly oxidized.
György, aerobic fermentation is redox neutral:
- Glucose + 2 NAD⁺ → 2 Pyruvate + 2 NADH
- 2 Pyruvate + 2 NADH → 2 Lactate + 2 NAD⁺
Combined:
- Glucose → 2 Lactate
How do you expect more glucose to solve the redox imbalance? It's the metabolic disinhibitions that do the trick, and controlled glucose exposure should be beneficial in the meantime.
Not shown above: about 1 ATP for every pyruvate or lactate formed. It's less than 1 because part of the glycolysis metabolites are diverted and lost.
But alternatively, it's possible to make use of the high flux to induce stress, leading to an overall helpful effect. Glucose must be paired with other compounds. Otherwise, you risk increasing the antioxidative capacity further.
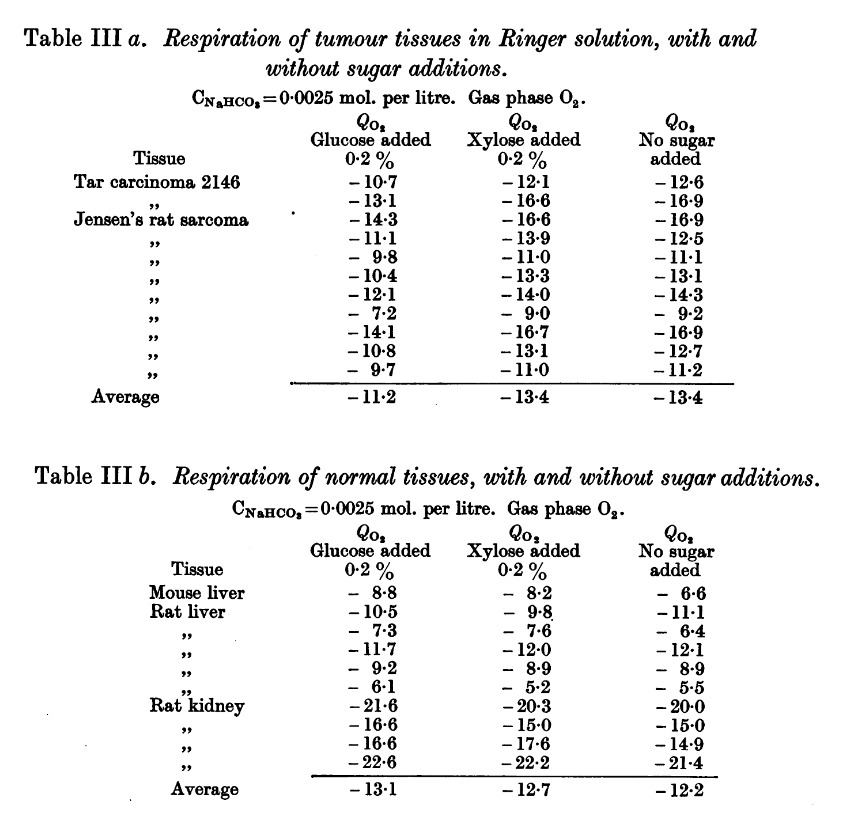
We have the glucose treatment that Ray mentioned, but it was used along with oxygen therapy. Without an adjuvant, in addition to improve the antioxidative capacity, glucose abundance could even reinforce any inhibition of respiration. Straight from Herbie's publication:
Observations on the Carbohydrate Metabolism of Tumours
-
@sneedful said in Vitamin C (IV route) doubles survival time in advanced pancreatic cancer patients:
@Mossy oh wait I said methylene blue fat thing that doesn't make sense cause u would be taking k2 mk4 instead. nvm. u get what I mean.
No worries.
-
Insulin given in isolation may actually increase the competition for glucose and divert some of it away from cancer cells. If released in response to glucose, whether we get lipolysis under control or not, we would still have the issue of ongoing fermentation and the need for something to change the cell behavior:
Another factor to consider:
Inside the hypoxic tumour: reprogramming of the DDR and radioresistance
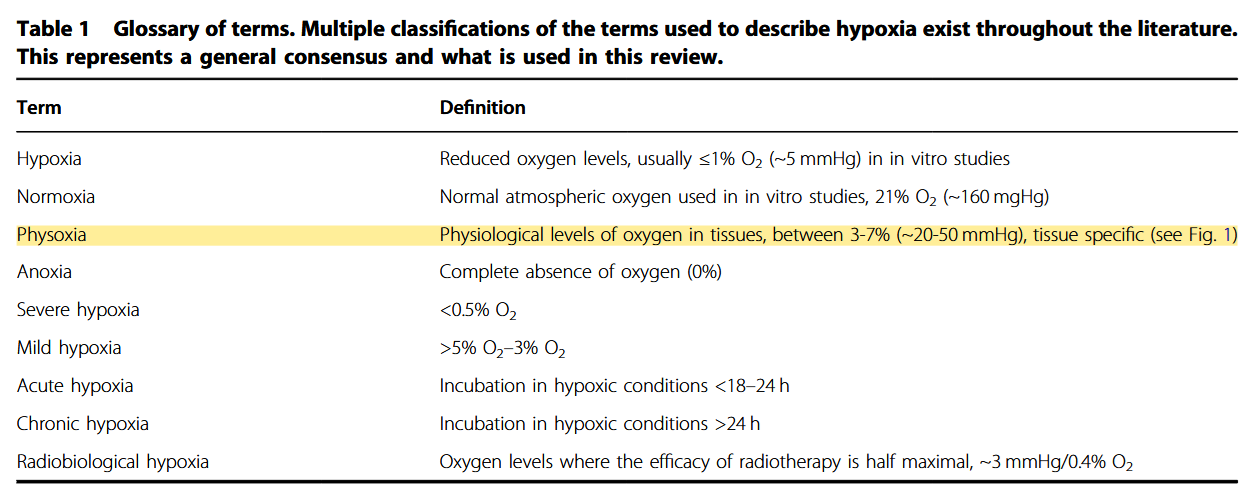
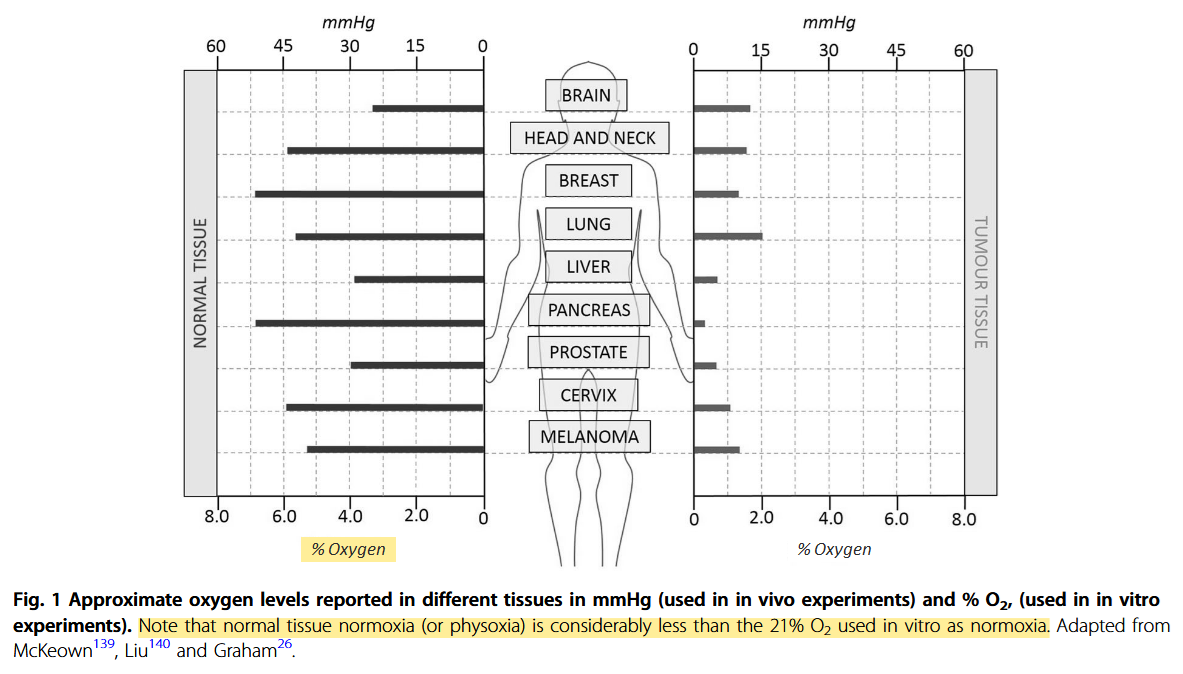
Additional information:
Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response
"Despite the many studies on tumour hypoxia, there is considerable confusion in the use of the terms “normoxia” and “hypoxia”. Oxygenation measurements in normal tissues show that they exhibit distinct normal ranges, which vary between tissues (Table 1)."
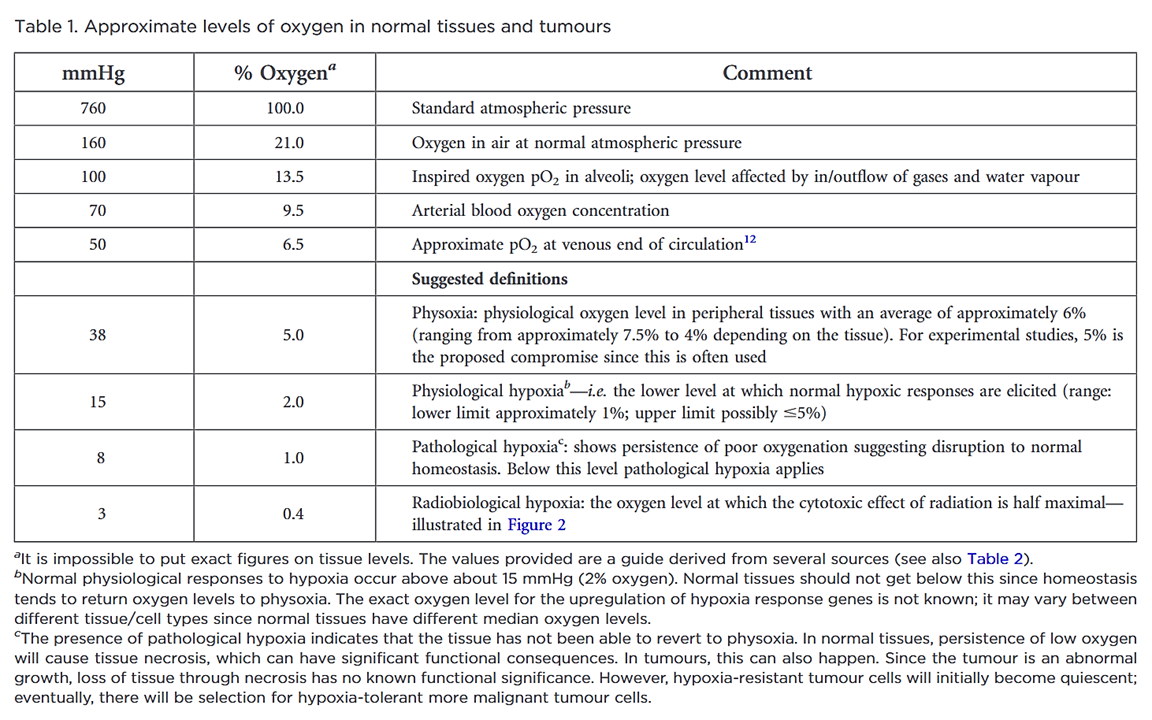
"However, “normoxia” is almost universally used to describe the “normal” oxygen levels in tissue culture flasks, i.e. about 20–21% oxygen (160 mmHg). Although this is not exact, as it is dependent on altitude and added CO2, for most situations, 20% is a good approximation. Despite the widespread usage of “normoxia”, this is far from an accurate comparator for peripheral tissue oxygenation. Even in lung alveoli, the oxygen level is reduced to about 14.5% oxygen (110 mmHg) by the presence of water vapour and expired CO2.[13] It drops further in arterial blood and, by the time it reaches peripheral tissues, the median oxygen levels range from 3.4% to 6.8% with an average of about 6.1% (Table 2).[13]"
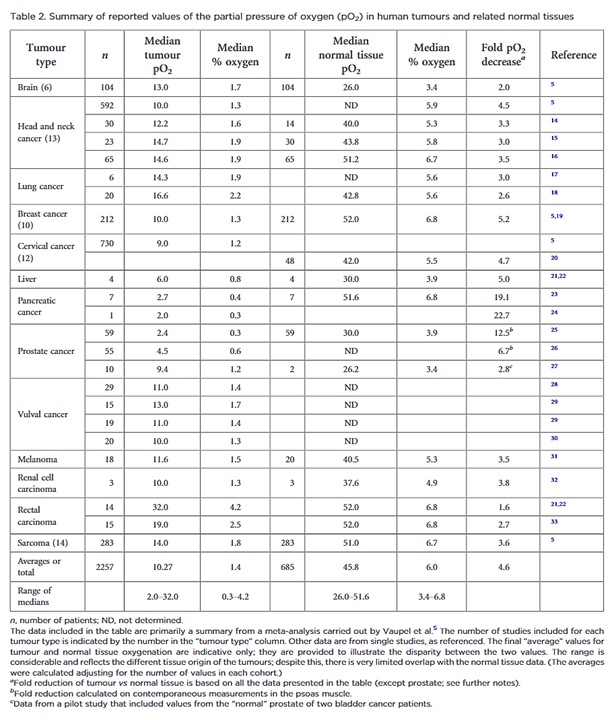
"In defining “pathological hypoxia”, there are no absolutes; however, the reality is that all tumours tend to have median tumour oxygen levels <2% and, within that estimation, individual measurements can vary from about 6% (very rarely) down to zero with a significantly marked skewing towards the lower end of this range; frequently, most values are well below 1.3% oxygen (10 mmHg), especially in the more hypoxic tumours."
Cells can maintain a surprisingly high rate of oxygen consumption in hypoxia:
Metabolic Profiling of Hypoxic Cells Revealed a Catabolic Signature Required for Cell Survival
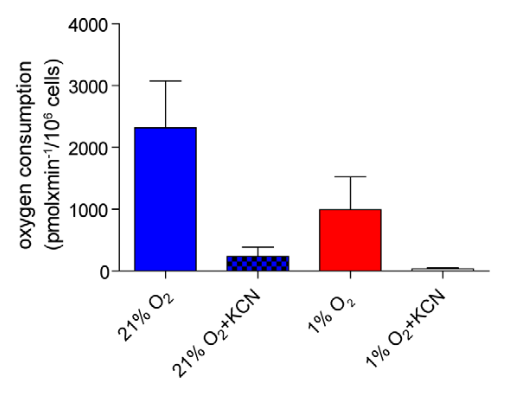
- Potassium cyanide (KCN) inhibits Complex IV (where most oxygen is supposed to be metabolized).
"[..]mitochondrial respiration can function at oxygen levels as low as 0.5% (Chandel et al., 1996; Rumsey et al., 1990)." (Luengo, 2021)
Despite the respiratory resistance, some cancer cells are found in severe hypoxia. You want them to respire normally with more glucose, but how, if little oxygen is available? They conserve oxygen by adopting an economical program. If these cancer cells keep up with the glucose flux and metabolize it liberally, it quickly depletes their oxygen and leads to further disturbances.
We can't turn our zealotry to fructose either:
Fructose contributes to the Warburg effect for cancer growth
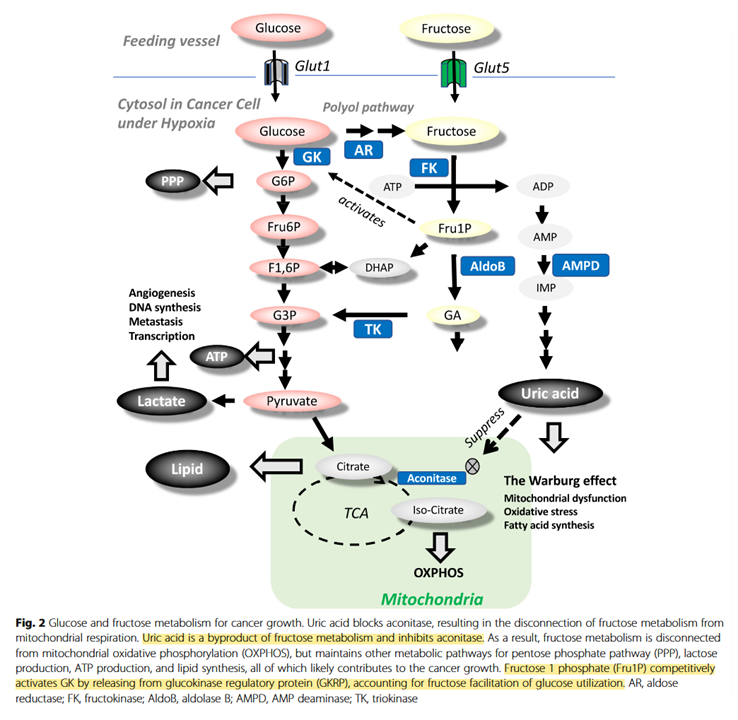
↳ Direct Spectrophotometric Determination of Serum Fructose in Pancreatic Cancer Patients
↳ Fructose induces transketolase flux to promote pancreatic cancer growth
"The enzyme TKT requires thiamine-derived mitochondrial NAD+ as a cofactor, and NAD synthesis can be disrupted with the thiamine analogue oxythiamine."
I forgot to list DHEA in Locketol:
Of interest, Heinz reported the following:
The Metabolism of Carcinoma Cells
"The carcinoma splits not only glucose to lactic acid, but also mannose, fructose and galactose, the velocity of the glycolysis being:
Toxin Relative speed Galactose 1 Fructose 2.5 Mannose 17 Glucose 18 Glucose is obviously attacked most rapidly and we might add the alpha-form of glucose as rapidly as the beta-form."
In comparison to abundance, glucose limitation is more supportive to increasing reliance on respiration and oxidative phosphorylation, because these are ways to maximize the efficiency in consuming a nutrient that can be depleted. Galactose is not metabolized fast enough to replace the mitochondrial yield. It's used in experiments when researchers want to make cells dependent on the mitochondrial metabolism.
Tumors can have regions with low levels of glucose, but pounding glucose in a state of metabolic derangement and hypoxia is more likely to intensify fermentation, and the typical structural abnormalities must follow.
These are not nutrient contraindications, more like anti-glorifications.
-
Why not make an impartial compilation of experiments on cancer and group them according to the effect of extra glucose (without additional factors) on cancer cells: supporting versus hindering proliferation? We can't go by the majority, but it's a starting place to question.
The line of thought would not be far from the idea that more glutamine should be delivered to cancer cells because they overconsume it to the point of depletion. Deficiencies can promote a more aggressive behavior, but it's not that we need the encouragement to load up on glutamine to compensate.
A condition of generalized scarcity is common, and this would call for the replenishment of many compounds. For example, immune cells can also compete with cancer cells for glucose, but also glutamine and other nutrients that may be rapidly used up.
Gerson's approach consisted of a moderate caloric intake, better distributed in smaller and more frequent meals, which leans in favor of a controlled exposure. If I was against dietary toxins, I wouldn't mention him often because the original therapy asked for minimal fats. Sometimes tumors have a person attached to them, and the person must be fed on occasion. Looking for potential downsides helps to identify and counteract them, but also avoids cults*. In case anyone knows superior dietary therapies to that of Gerson, I wouldn't mind relegating it, no matter how unconventional.
- *Reflected on a thread such as our "Can glucose loading cure everything?"
It would be good to know how relevant fructose-1-phosphate would be on tissues that don't express ketohexokinase or glucokinase (a variant of hexokinase). Also, how high uric acid levels have to be to affect aconitase.
But it makes more sense to seek local nutrient repletion when you can target the misbehavior or exploit it.
In the previous experiment, they first manipulated Hypoxia-inducible Factor (HIF) to lift some of the metabolic inhibitions and only then pound glucose with insulin, intending to provoke oxidative stress. Otherwise, the effect was insignificant.
It's not difficult to picture glucose lessening the oxidative burst from vitamin C therapy when not much else is done to manipulate the fermentation pattern.
NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications
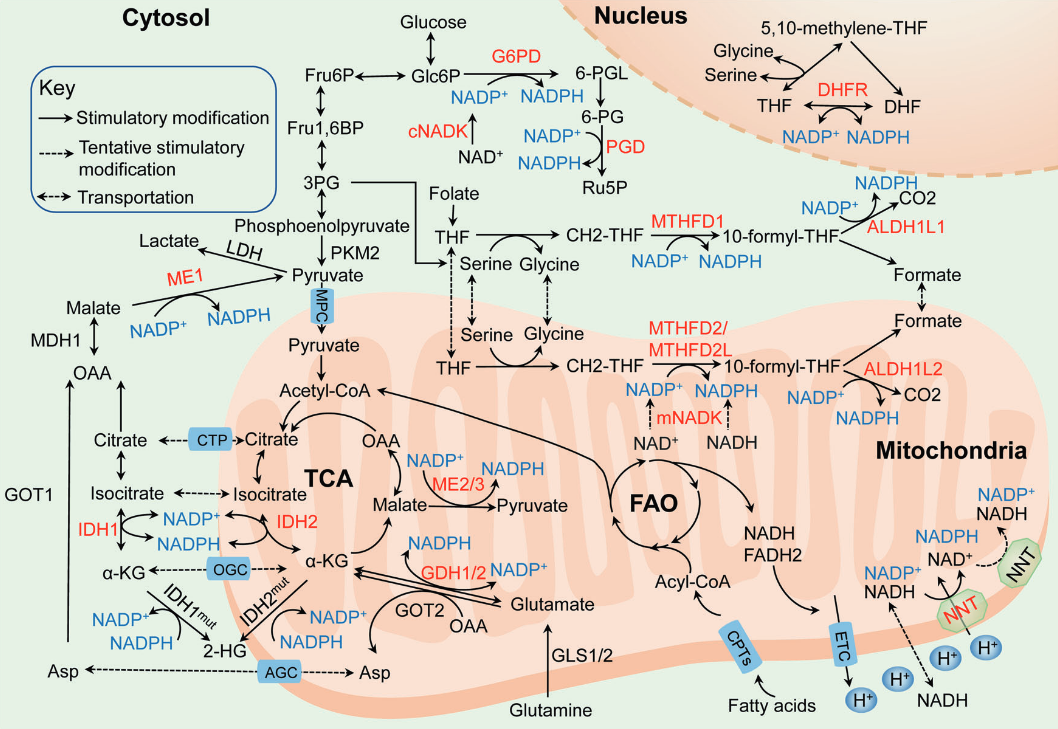
NADPH-producing enzyme Origin of main substrate G6PDH Glucose PGDH Glucose ME Glutamine, glucose IDH Glutamine MTHFDH Glucose ALDH Glucose GDH Glutamine NNT Glutamine, fatty acids, glucose Glucose may also spare glutamine in antioxidation.
By the great Matthew Vander Heiden and Sophia Lunt:
Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation
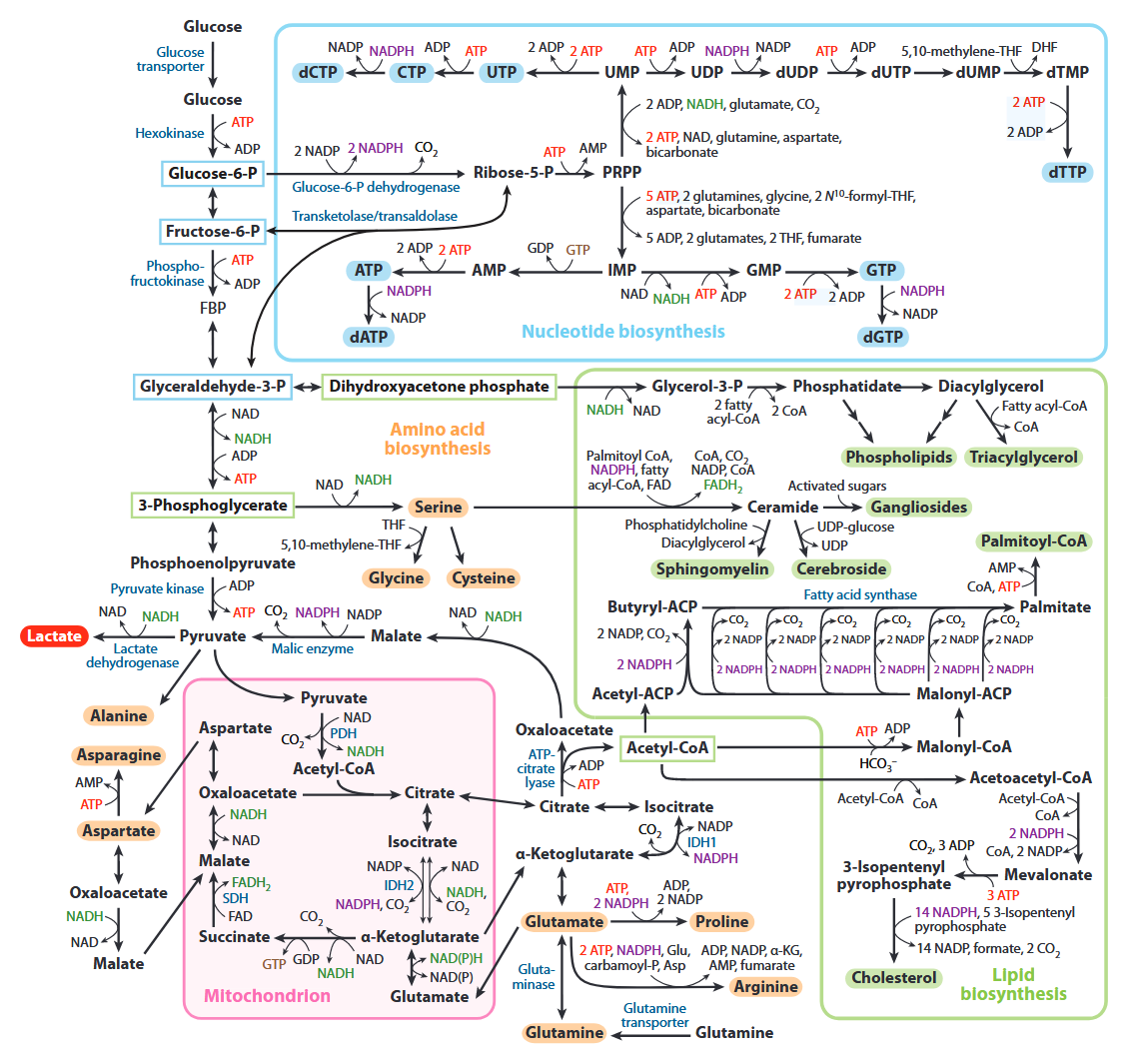
All of the carbons that occur in nucleotides can conceivably come from glucose. It's absurd.
Carbon source Derivation Carbons donated Ribose Glucose →→ ribose 5 Aspartate Glucose →→ pyruvate → oxaloacetate → malate → asp 3 Formyl-THF Glucose →→ serine →→ formyl 2 Glycine Glucose →→ serine → glycine 2 Hydrocarbonate Glucose →→ carbon dioxide → hydrocarbonate 1 Methylene-THF Glucose →→ serine →→ methylene 1 Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides
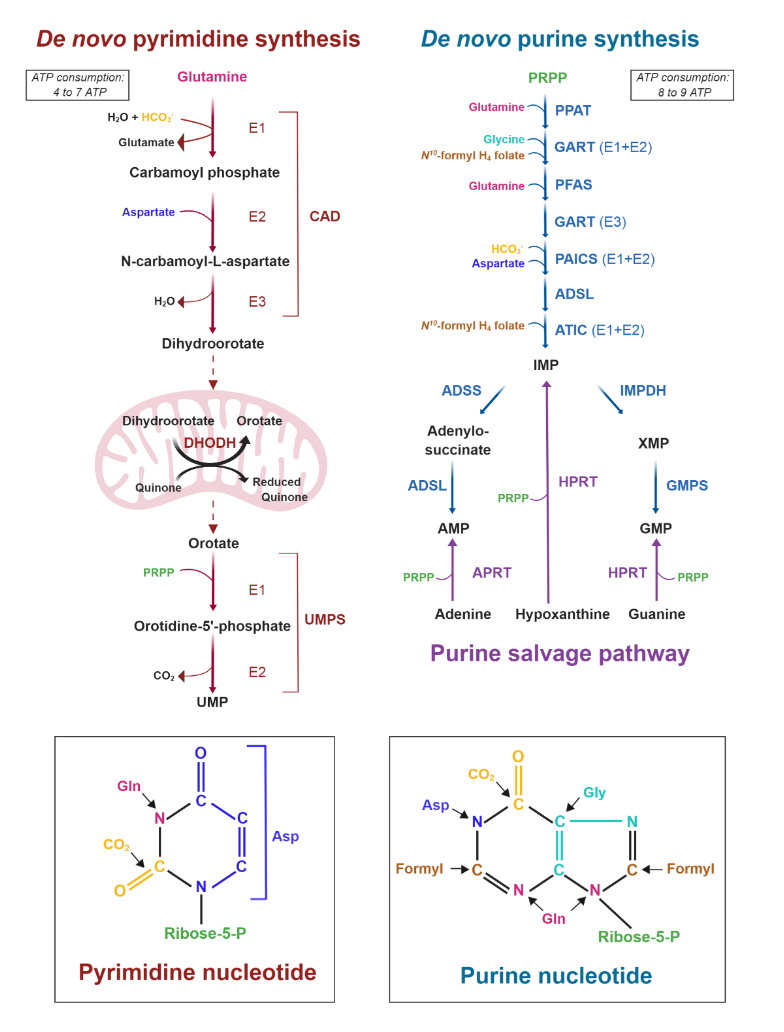
It's also evident that the base of phospholipids and triglycerides can be derived from glucose in the form of glycerol.
Ceramide needs serine, which can come from glucose as well.
In addition, serine accepts the sulfur from homocysteine to yield cysteine, and then glutathione.Expanding on the fates of glucose, pyruvate kinase (PK) is the last enzyme of glycolysis, responsible for the following simplified reaction:
- Phosphoenol-pyruvate (PEP) + ADP –(PK)→ pyruvate + ATP
PK has 4 variants:
Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis
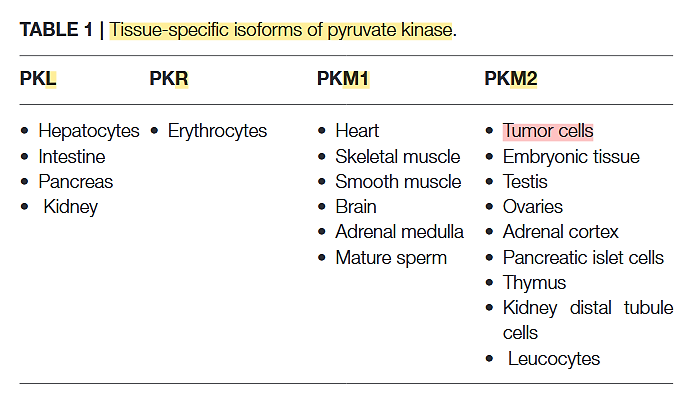
The PK-M2 prevails in tumor cells and can occur as a dimer or as a tetramer:
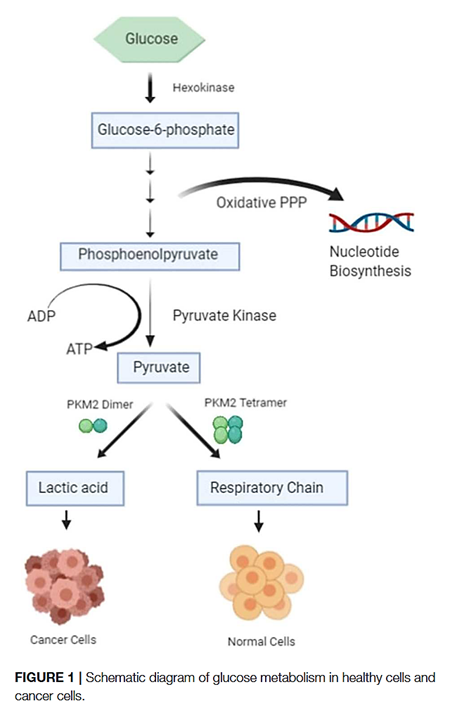
- PK-M2 dimer is hypoactive → Less pyruvate and ATP
- But the lower rate of conversion creates a metabolite congestion that spills over to the branches of glycolysis → More biosynthesis and antioxidation
⠀
- But the lower rate of conversion creates a metabolite congestion that spills over to the branches of glycolysis → More biosynthesis and antioxidation
- PK-M2 tetramer is hyperactive → More pyruvate and ATP
They differ in low and high affinity for the substrate PEP and give (tumor) cells more flexibility to rely on either state depending on needs for biosynthesis or energy. Excess ATP inhibits multiple metabolic steps and can be counterproductive.
Oxidative stress inhibits the enzyme, which is a convenient way to recreate the hypoactive situation above.
Expressing PK-M2 is an advantage, not a requisite.
- No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis
- M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth
The cell can adopt different strategies in diverting glucose from glycolysis:
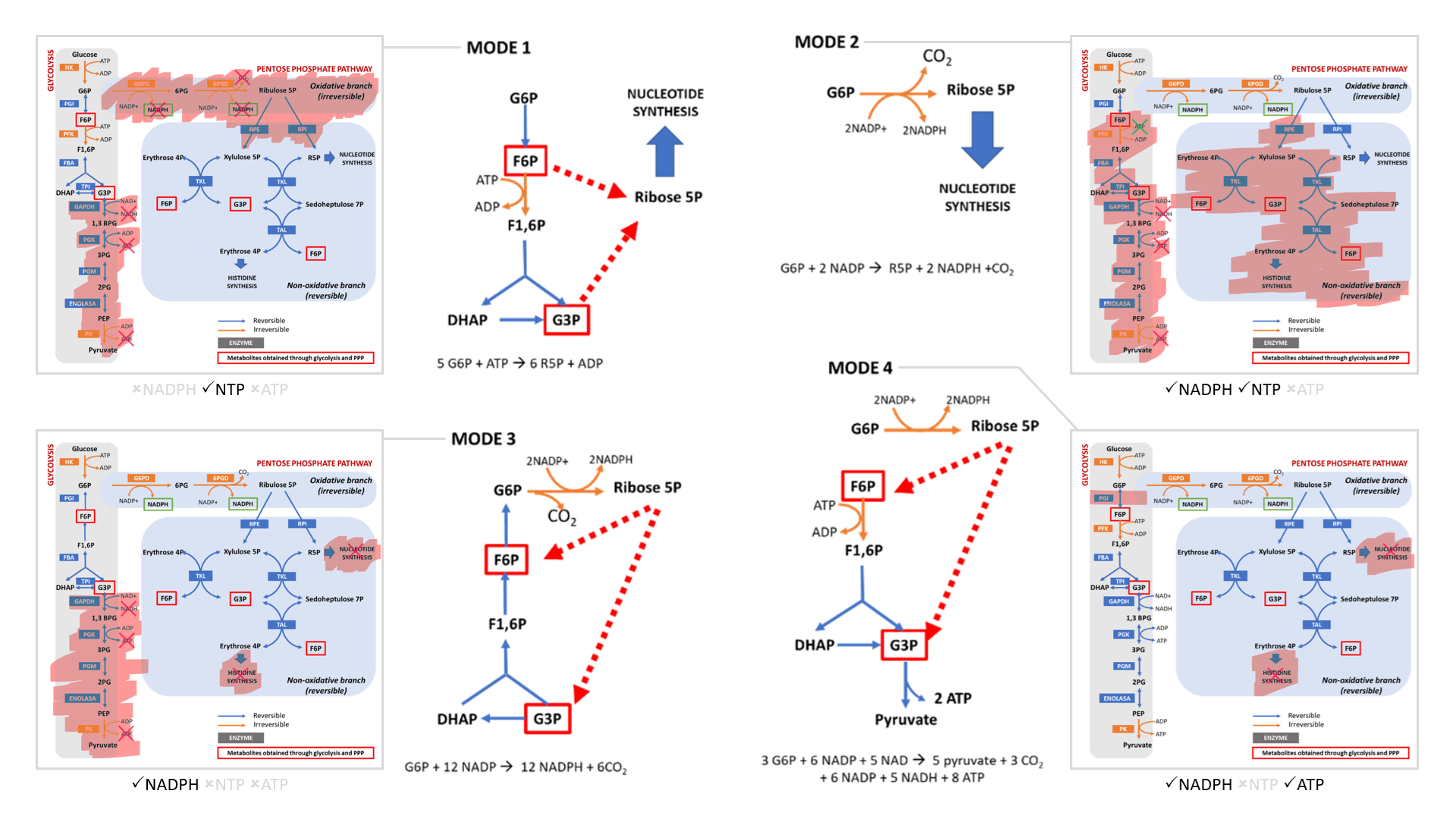
- Modes 1 and 2 result in sudden loss of carbons as ribose for nucleotides, without or with decrapoxylation.
- Mode 3 represents a cycle for continuous production of NADPH until complete decrapoxylation. It may be an overlooked source of carbon dioxide. Note that it bypasses a reaction that's treated as irreversible (F6P ↶ F1,6P).
- Only mode 4 yields pyruvate with the net gain of regenerated ATP.
Rather than serving to normalize the redox state of cancer cells, glucose metabolism can easily go from redox neutral in straightforward fermentation to reductive in case of diversion with return.
If we arrive on pyruvate [3C]:
The metabolic fates of pyruvate in normal and neoplastic cells
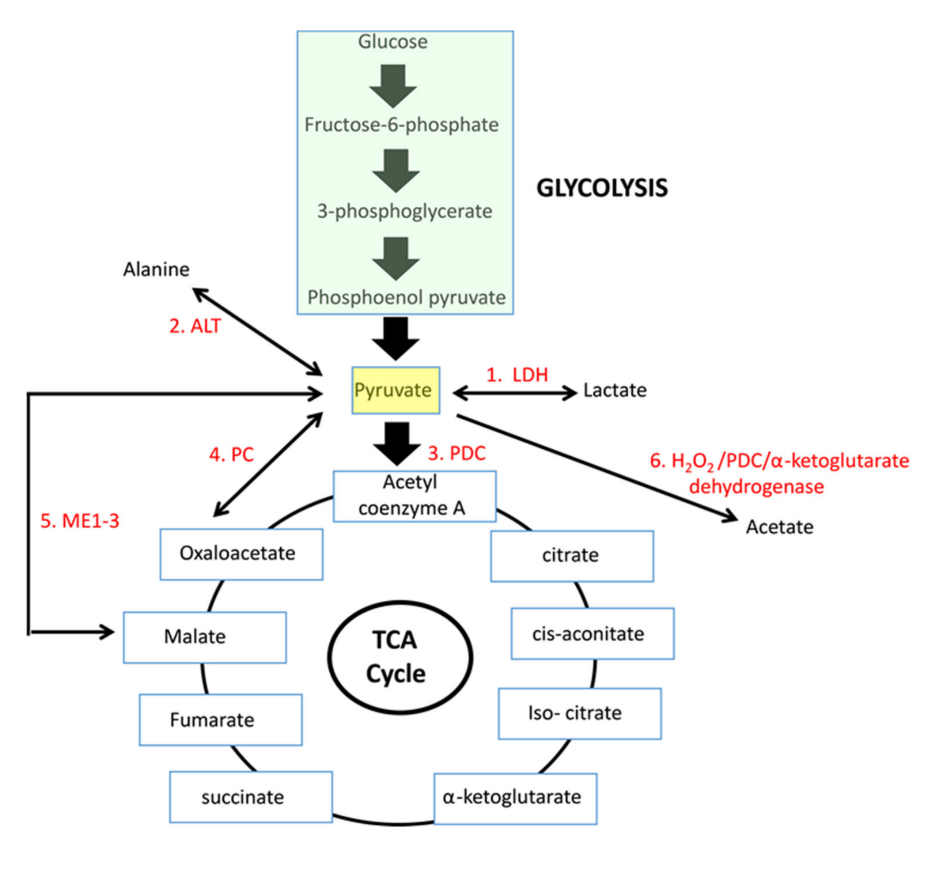
Enzyme Reaction description Main changes Km [low value: high affinity] 'PDC' Oxidation to acetyl(CoA) [2C] –2H –CO₂ 0.02 mM (0.005-0.043 mM) LDH Reduction to lactate [3C] +2H 0.1 mM (0.034-0.630 mM) PC Crapoxylation to oxaloacetate [4C]* +HCO₃⁻ (+ATP) 0.265 mM (0.23-0.30 mM) ME Crapoxylation to malate [4C] +2H +HCO₃⁻ 0.75 mM (GPT or) ALT Amination to alanine [3C] +NH₄ 2.8 mM (0.07-12.50 mM) N/AOxidation to acetate [2C] ? +H₂O₂ Each has cytosolic and mitochondrial forms, with the exception of PDC. Pyruvate import (and not just its oxidation) may be impaired, and pyruvate is formed in amounts that are only comfortably processed by LDH.
Glutamate can be oxidized (through GDH) or transaminate to produce ketoglutarate. Pyruvate (through ALT (⇈)) can accept the amino group of glutamate to become alanine and yield ketoglutarate. So, pyruvate offers an alternative way to metabolize glutamate. Lactate is known to be elevated in cancer, but we now know that alanine is somewhat elevated too.
I'm aware of the ethyl-pyruvate experiments, but the picture is more complicated than apparent, in special for pyruvate derived from glucose.
Conversely (and beyond acidification):
But to get to this condition naturally, you'd need disturbing levels and the associated concerns to achieve it.
I have to stress that this is not vilify dextructose or levilose, but to put things into perspective. If we are extra cautious with folate to the point of discouraging its use preventively (!), we can't brush these aspects off, including the fact that glucose is a major carbon donor that keeps the folate cycle running, with glycolysis and its branches being overactive in cancer. And if we have antifolates as cancer therapies, folate should be present in enough quantities to matter as a carrier.