Fats functions and malfunctions
-
Fats functions and malfunctions
*) Thyroid affects mitochondrial energy
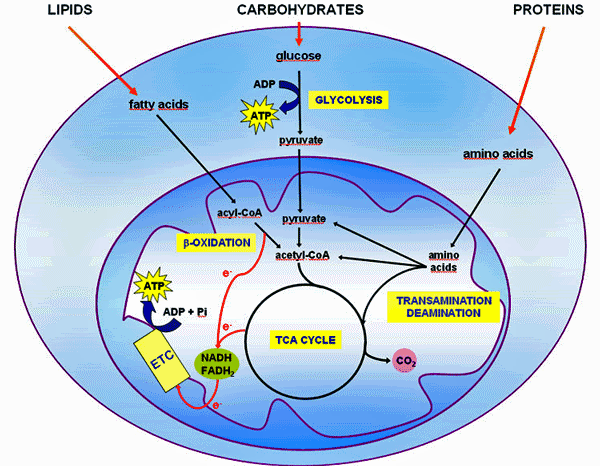
Cellular respiration takes place in the mitochondria. Mitochondria are often described as the "powerhouse" of cells, as they contribute to most of the cellular ATP production through β-oxidation, the Krebs cycle, and the respiratory chain. ATP is the ubiquitous energy molecule used in a very large number of chemical reactions in metabolism. (1)
*) Nutrient plasticity for TCA cycle in mitochondria
Figure 1: when fats or carbs enter the Krebs cycle (TCA)
*) **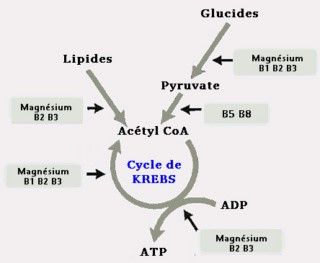
*) A three-way energy synthesis: Carbs and lipids are the two preferably choices for converting energy (ATP). Cells make their own decisions according to needs (adaptation process).
Figure 2: A three-way energy synthesis
When there is a metabolic overload or dysfunction, excess hydrogen peroxide (ROS) occurs. “ROS induces a shut-down of metabolic transporters and leads to impaired mitochondrial problem, like insulin sensitivity and low-grade inflammation.” (Chris Masterjohn in Oxidants mediate adaptations to cellular energy overload).
Note: A two-sided, self-perpetuating problem. When cellular energy overloads mitochondrial capacity to handle energy supply, the electron transport chain makes more reactive oxygen species.Figure 3: cellular energy overload induces ROS
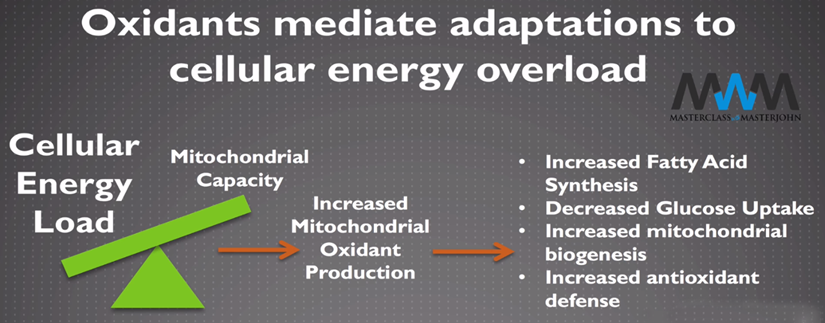
Chris Masterjohn – MWM video
It's not the fault of ROS, it's not a connection fault or a defect in the electron (ion) pairing; it's not the fault of glucose transporters or a voltage defect in ion channels; it's not the fault of anything except the context: what you have is an abnormal physiological response to a pathological context due to a wild energy overload in the general metabolism (in the whole body system). Too much fuel at the same time.
It's a problem that lies in the regulation of cellular energy metabolism, according to its capacity/rate to produce ATP. Expressed more simply: Are you able to burn the amount of energy supplied? If the influx exceeds capacity, you must either increase your fuel consumption (exercise/heat) or reduce your calorie intake briefly. In the last case, eat less (but still 80% of your daily average, editor's note, with a sufficient protein intake, like 1.2 g/kg weight).
Decoding: Not everyone will be able to drink a cup of coffee, as first thing in the morning, followed by a large glass of orange juice, accompanied by cereal and milk. Why? Bad combination for managing your blood sugar. Not really a bad function of your pancreas but an excessive balanced intake. Not everyone burns carbohydrates on the same way. Need to modify the carbohydrate management, and the association of macronutrients (order matters). We need saturated fats at breakfast (steroid hormones).*) Mitochondrial β-oxidation of fatty acids generates acetyl-coA, NADH and FADH2. Acyl-coA synthetases catalyze the binding of fatty acids to coenzyme A to form fatty acyl-coA thioesters, the first step in the intracellular metabolism of fatty acids. L-carnitine system facilitates the transport of fatty acyl-coA esters across the mitochondrial membrane. (2)
*) What kinds are preferably oxidized for energy?
Our body metabolizes preferably saturated fats and monounsaturated fats (olive, macadamia, and avocado). Your liver and intestines make about 80% of the cholesterol you need to stay healthy. Only about 20% comes from the foods you eat. (3)*) In tissues with high energy requirement, such as heart, up to 50–70% of energy, in the form of ATP production, comes from fatty acid (FA) beta-oxidation. The name beta-oxidation comes from the fact that the beta carbon of the fatty acid is oxidized generating a carbonyl group. The acetyl-CoA thus generated enters the Krebs cycle (TCA). PMID: 27080715
Schematic representation of the mitochondrial fatty acid β-oxidation. Long-chain fatty acids need to be transported into the mitochondria by an active transport system. Because fatty acids in the cytoplasm are activated as acyl-CoA esters, they must be bound to carnitine for transport across the mitochondrial membranes. In contrast, medium- and short-chain fatty acids do not need an active transport system and can easily undergo β-oxidation. (4)*) Mitochondrial Metabolism of Fatty Acids
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073799
Mitochondria are the powerhouse of the cells, generating up to 90% of the energy within a cell in the form of adenosine triphosphate (ATP). There is a close connection between fatty acid metabolism and mitochondria, involving a considerable number of cellular processes that go well beyond mitochondrial fatty acid metabolism. Fatty acids are essential for ATP and energy production, and are therefore highly relevant in the regulation of energy homeostasis. The processes of β-oxidation, linked to ATP production, and mitochondrial fatty acid biosynthesis (mtFAS) are both localized in the mitochondria. This last pathway, in particular, produces molecules that are used as cellular structural components for post-translational modifications of proteins and in signaling cascades.&1. Mitochondrial β-Oxidation
Mitochondrial β-oxidation is essential for energy production from fat. Short- and medium-chain fatty acids can cross the mitochondrial double membrane without the need for an active transport system and subsequently participate in the β-oxidation cycle [1]. By contrast, longer-chain fatty acids cannot passively cross the mitochondrial membrane [2]; for this purpose, the long-chain fatty acids are covalently bound by carnitine palmitoyltransferase I (CPT1) located on the mitochondrial outer membrane. (see figure 1). (5)*) Oxidized fats
Oxidizing fatty acids isn't harmful as long as the liver is functioning properly. Keep in mind that stored fat has a limited lifespan. There's a turnover. A problem arises when we overload our metabolism. The amount of oxidized fatty acids (OxLDL) then becomes a problem because the level is too high.*) What are the benefits of eating good saturated fats?
Saturated fats are fundamentally pro-metabolic. On a cellular level, they oppose cytoplasmic glycolysis and promote mitochondrial oxidative phosphorylation.
Saturated fats stabilize proteins, other lipids and other cellular components. This is because they are molecularly more stable / less fragile in a body at 98° F (or 37° C). A stress-adaptive pathway will avoid a degraded environment, provided there aren’t too many stressors: metabolic (low ATP), excitatory (Ca++ or EMF radiation), osmotic (Nitric Oxide) and redox (ROS and lack of antioxidants). So, we’ll avoid cell proliferation and induced apoptosis if we balance the activities well. Otherwise, all stress-adaptive pathway in excess (when repeated) will induce insulin resistance, increase proliferation (apoptosis or cancer) and reduce mitochondrial efficiency. -
Sources and References
- Thyroid affects mitochondrial energy
https://mirzoune-ciboulette.forumactif.org/t2018-comment-booster-votre-energie#29552
=> How to boost your energy level (In French, translator needed) - Mitochondrial β-oxidation of fatty acids
https://doi.org/10.1016/j.mito.2018.02.009 - What kinds are preferably oxidized for energy?
Our body metabolizes preferably saturated fats and monounsaturated fats.
https://www.health.harvard.edu/heart-health/how-its-made-cholesterol-production-in-your-body - Schematic representation of the mitochondrial fatty acid β-oxidation
https://encyclopedia.pub/entry/9223 (figure 1). - Short- and medium-chain fatty acids can cross the mitochondrial double membrane without the need for an active transport system
https://encyclopedia.pub/entry/9223#4_Metabolic_Flexibility_and_Interconnection_between_Mitochondrial_Oxidation_and_Fatty_Acid_Biosynthesis
- Thyroid affects mitochondrial energy
-
Additionnal info:
*) Conception erronée au sujet des propriétés physiques et fonctionnelles des acides gras saturés (Misconception about the physical and functional properties of saturated fatty acids)
https://mirzoune-ciboulette.forumactif.org/t2073-conception-erronee-au-sujet-des-proprietes-physiques-et-fonctionnelles-des-acides-gras-satures
Tuons un mythe : les graisses polyinsaturées ne sont pas utiles pour assurer la perméabilité/osmolarité des membranes. La théorie relative à la « membrane » est inadéquate. Dire que les oméga-3 rendent les membranes cellulaires plus fluides et donc « mieux fonctionnelles » est au mieux un conte de fée biologique.
Traduction:
Let's dispel a myth: Polyunsaturated fats are not useful for ensuring membrane permeability/osmolarity. The "membrane" theory is inadequate. Claiming that omega-3s make cell membranes more fluid and therefore "better functioning" is, at best, a biological fairy tale.
*) How the Oils In Your Diet Are Aging Your Skin.
Dr. Peat goes on to explain what this rancidity (oxidation process) does:
"The free radicals produced in this process react with parts of cells, such as molecules of DNA and protein, and may become attached to those molecules, causing abnormalities of structure and function."
https://raypeat.com/articles/articles/unsaturated-oils.shtml
*) Les besoins réels en AG polyinsaturés surestimés ?
https://mirzoune-ciboulette.forumactif.org/t1581-les-besoins-reels-en-ag-polyinsatures-surestimes
=> Are the real needs for polyunsaturated fatty acids overestimated?
*) Toxicité des AG polyinsaturés en excès
https://mirzoune-ciboulette.forumactif.org/t1685-trop-domega-3-deprime-le-systeme#21200
Trop d'acides gras insaturés dépriment le système (Too many unsaturated fatty acids depress the system).
*) Aging remodels membrane phospholipids
Phospholipids contribute to the fluidity and flexibility of membranes, allowing for membrane dynamics and cell movements.
Figure 4: Phospholipidic membrane
Phospholipidic membrane
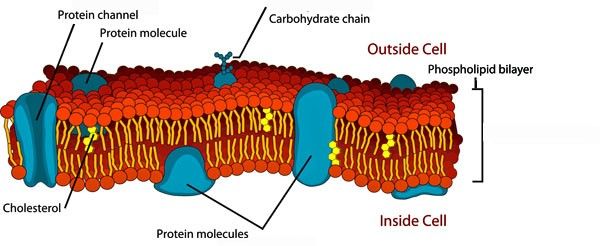
Senescence remodels the hydrocarbon tail of membrane phospholipids
Phospholipid bilayers are critical components of cell membranes. The lipid bilayer acts as a barrier to the passage of molecules and ions into and out of the cell. However, an important function of the cell membrane is to allow selective passage of certain substances into and out of cells.
Phospholipids tail length is modified when aging (oxidized) and this change affects permeability by changing the membrane thickness. The aging brain undergoes significant remodeling of lipid composition, as well.