Mead Acid, desaturases and accelerating PUFA(EFA) depletion
-
“The enzyme that produces the Mead fatty acid is strongly inhibited by PUFA seed oils (less strongly by fish oils), and so the presence of the Mead acid in the tissues is taken as evidence that the animal is suffering damage resulting from the absence of PUFA. The Mead acid happens to have some valuable anti-inflammatory effects, and is associated with many biological advantages, but research in that direction is prevented by the lack of funding.” Ray Peat
When Ray mentions the ability of EFAs to suppress the production of Mead Acid, he is talking about the effects they have on desaturases, and although ω-3 has the ability to suppress Mead acid this would probably only happen if ingested in large quantities, the biggest reason for the increase in Mead acid production is ω-6 restriction.
So first of all, you should know that desaturases "add 1 double bound" and elongases "add 2 carbons". Let's take stearic acid as an example, it has an 18-carbon chain and because it is saturated it has no double bounds, so we classify it as 18:0. When it is used as a substrate by the enzyme ∆-9 desaturase (D9D) a double bound is added and it becomes 18:1, so stearic acid (18:0) has been converted into the monounsaturated oleic acid (18:1). The logic is similar for elongases, if 18:1 is used as a substrate by an elongase it would be converted into a 20:1 fatty acid.
To produce Mead's acid, oleic acid (18:1ω-9) is the substrate for the enzyme ∆-6 desaturase (D6D), which transforms it into 6-9-octadecadienoic acid (now 18:2ω-9) which then undergoes the effects of an elongase and is converted into 8-11-eicosadienoic acid (now 20:2ω-9). The last step after this is to pass through ∆-5 desaturase (D5D), converting it into Mead acid(5-8-11-eicosatrienoic acid) with 3 double bounds (20:3ω-9).
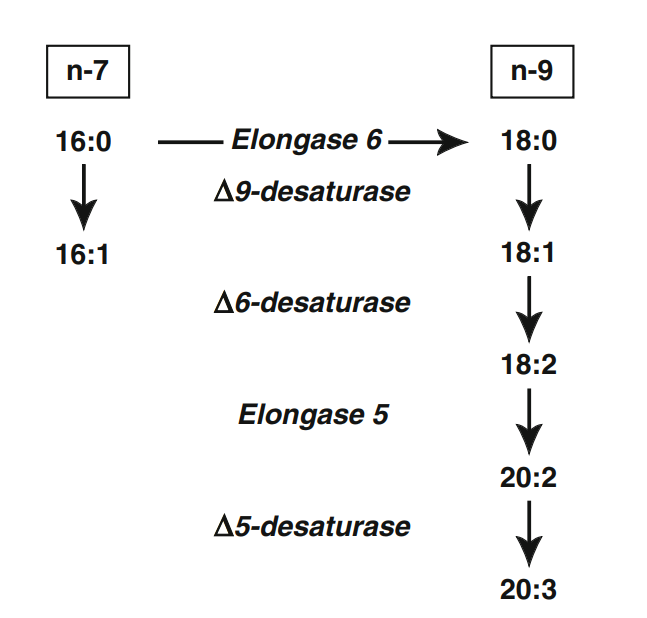
The strong inhibition of Mead acid production by EFAs is due to their affinity for the D6D enzyme. α-linolenic acid has priority, followed by linoleic acid and then oleic acid.
The increase of the affinity of the ∆6-desaturase from oleic to linoleic and α-linolenic acid, as well as the correlative increase of the rate of their desaturating reactions explain the predominance of the polyunsaturated acids of the linoleic family or α-linolenic family over the acids of the oleic family in animals fed a complete diet.
For this reason, I can only see two ways of producing Mead's acid in larger quantities:
-
By elimination: As Ray suggests, eliminating "essential" fatty acids (focus on eliminating ω-6) from the diet and accumulated in the body, thus releasing D6D for oleic acid.
-
By competition: A greater flow of oleic acid in "unusual" situations. Just eating more MUFA won't make you produce more Mead's acid (unless perhaps you were already depleted of EFAs), but there are studies on rats that show that fasting for 3 days and refeed even with an ordinary diet will make the level of Mead's acid rise. The supposed reason? Fasting partially suppresses the desaturases and there is an overcompensation in refeed, apparently for brief moments there is an insufficiency of EFAs and an abundance of MUFAs such as Oleic, available to desaturases.

Control(normal), control-induced(fasting+refeed)
Accelerating pufa depletion through desaturases
In addition to the affinity factor, different macros exert different effects on desaturases, which in theory makes it possible to accelerate EFA depletion by manipulating desaturases. If we consider cystic fibrosis(CF) as an example, Mead's acid is common and is not always due to super low levels of linoleic acid (although they are almost always low), but rather to an increase in the expression of desaturases.
CF: Among these abnormalities are increases in the levels of n-7 and n-9 fatty acids, particularly palmitoleate (16:1n-7), oleate (18:1n-9), and eicosatrienoate or mead acid (20:3n-9)
Work from our laboratory provided correlative data suggesting that these metabolic changes were due to increased expression and activity of fatty acid desaturases ( 23, 24 ). The current study demonstrates that treatment of CF cells with exogenous DHA reverses the metabolic changes by suppressing expression of these enzymes. Specifically, DHA decreases expression of 5- and 6-desaturases.
I found a study in which they accelerated "essential" fatty acid deficiency by inducing D9D, fasting for 3 days + 4 days of refeed.
Biochemical indicator of "essential" fatty acid deficiency is a triene:tetraene ratio (20:3n-9:20:4n-6) greater than 0.4, and below is the difference between EFAD (fat-free diet without fasting) vs EFAD-Induced (fasting+refeed with fat-free diet) over 3 weeks.
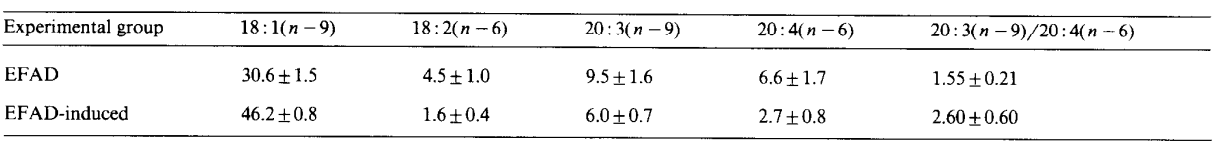
Comparing the two, EFAD has a T:T ratio of 1.55 while EFAD-induced has a ratio of 2.60. The former's ratio seems to be more influenced by the increase in Mead Acid (20:3) with almost 60% more compared to EFAD-Induced, which seems to have a high ratio due to the depletion of Linoleic (18:2) and Arachidonic (20:4). This T:T ratio doesn't reflect all the tissues in the body, so this highlight is from the serum.
Do you remember that different macros influence enzymes in different ways? So.
Example 1:
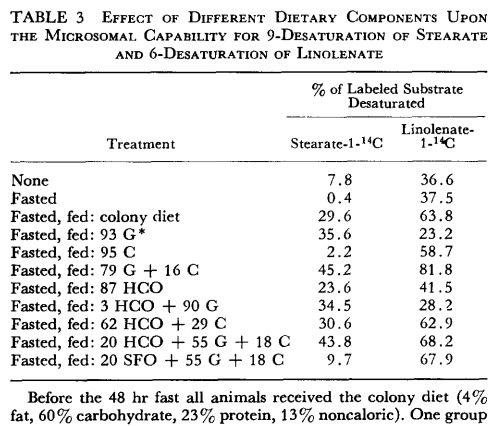
Obs.: G= Glucose, C = Casein, HCO = Hydrogenated coconut oil, SFO = Sunflower Oil.Example 2:
While fasting is not "peaty" I have the impression that a similar or superior result can be achieved with a protein restriction replacing these 3 days of fasting. Protein restriction seems to have the same effects on desaturases, combined with carbohydrate increasing D9D and low fat (close to 0 PUFA) might be better.
In monkeys:
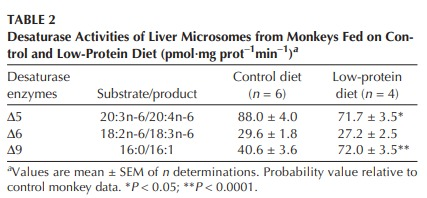
In rats:
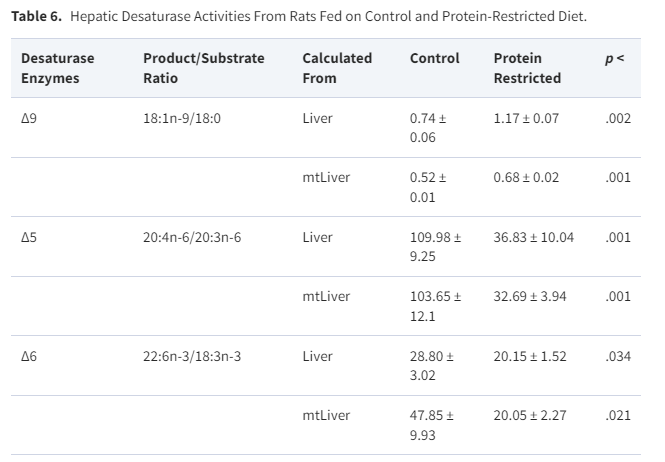
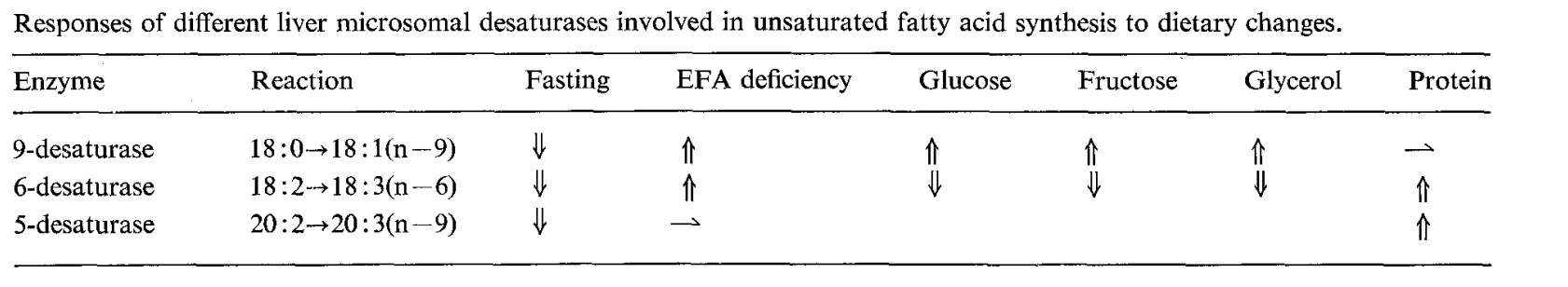
In the present work a time-course study with young rats fed on a low-protein diet was an original approach for protein restriction, providing evidence that ∆-9 desaturase activity is impaired, as indeed are ∆6 and ∆5 desaturase activities, during growth under such a dietary regimen.
The effects of this cyclical approach, which accelerates the depletion of EFAs, are more localized:
The mol% of 20 : 3(n - 9) in serum was not increased by ∆9 desaturase induction and the 20 : 3(n - 9) to 20 : 4(n - 6) ratio was only modestly increased. The effects of ∆9 desaturase induction were even more attenuated in tissues other than the liver.
The liver is not the only tissue that shows changes in fatty acid composition. It has been noted, however, that the extent of change is much more profound in liver during the limited 48 hr refeeding period. In Table 5 it is seen that adipose tissue (epididymal fat pad), heart tissue, and the remaining carcass also reflect the variations noted with liverr, but the extent of change is less in the period studied.
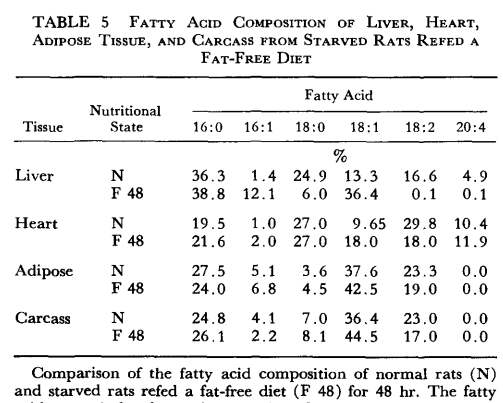
So I think you can still expect a general acceleration on a diet with <0.8% linoleic acid, since even those on the control diet produced a small amount of Mead's acid, I suppose that some of it is transported out of the liver and what would take 4 years could be reduced to less due to competition, who knows.
The above studies showed that the concentrations of other PUFAs, such as ARA, EPA and DHA, decreased due to their partial displacement by Mead Acid(MA). The results suggest that MA competes with other long chain PUFAs in PUFA metabolism, and that the presence of MA is not solely due to the decrease in ARA in patients with EFA deficiency. The competition of MA with other PUFAs, especially ARA, is a main mechanism underlying its various physiological and pathological activities, as described below.
Besides, considering that simple approaches can have this influence opens up new possibilities for experimentation: would restricting calories a little on restriction days also increase overcompensation in refeed? And if I use niacinamide/aspirin on restriction days to decrease de novo lipogenesis, what would be the effect?
References
DAVID W. ALLMA, DOROTHY D. HUBBARD, and DAVID M. GIBSON. "Fatty acid synthesis during fa t-free refeeding of starved rats"
Hui Gyu Park, Matthew G. Engel,Kyle Vogt-Lowell, Peter Lawrence, Kumar S. Kothapalli, and J. Thomas Brenna. "The Role of Fatty Acid Desaturase (FADS) Genes in Oleic Acid Metabolism: FADS1 Δ7 desaturates 11-20:1 to 7,11-20:2"
J B Lefkowith. "Accelerated essential fatty acid deficiency by ∆9 desaturase induction: dissociation between the effects on liver and other tissues".
CAROL A. INKPEN, ROBERT A. HARRIS and FORREST W. QUACKENBUSH. "Differential responses to fasting and subsequent feeding by microsomal systems of rat liver: 6- and 9-desaturation of fatty acids"
Rodolfo R. BRENNER "The oxidative desaturation of unsaturated fatty acids in animals"
María C. Marína, Héctor M. Pucciarellib, and María J.T. de Alaniza. "Liver Desaturase Activities and FA Compositionin Monkeys. Effect of a Low-Protein Diet".
Victoria Ayala, Alba Naudí, Alberto Sanz, Pilar Caro, Manuel Portero-Otin, Gustavo Barja, Reinald Pamplona. "Dietary Protein Restriction Decreases Oxidative Protein Damage, Peroxidizability Index, and Mitochondrial Complex I Content in Rat Liver"
Hiroshi Kawashima and Katsuhiko Yoshizawa. "The physiological and pathological properties of Mead acid, an endogenous multifunctional n-9 polyunsaturated fatty acid"
Sarah W Njoroge, Michael Laposata, Waddah Katrangi, Adam C Seegmiller. "DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity"
Kelly F Thomsen, Michael Laposata, Sarah W Njoroge, Obi C Umunakwe, Waddah Katrangi, Adam C Seegmiller. "Increased elongase 6 and Δ9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis"*
-
-
@TexugoDoMel great post! Is there anything protein restriction can't do?
It even helps with PUFA depletion.
At this point I count around 10 top tier mechanisms, all of which would be worth pursuing on their own, through which protein restriction acts.
I think protein restriction is a great addition to the "peat diet", but even that the late peat had figured out. -
@Mauritio What do you think is a good protein restriction goal? How much per day do you think would be optimal?
-
@forty said in Mead Acid, desaturases and accelerating PUFA(EFA) depletion:
@Mauritio What do you think is a good protein restriction goal? How much per day do you think would be optimal?
Maybe 40-60g depending on your caloric requirements, aim for 10% protein or less.
But more importantly control for cysteine and methionine intake.
I think 0.12% of both is a good goal.
Or 10.4mg/kg/day, which they successfully used in a human study.It is a bit of work to calculate this, but it helps a lot and if you did it once you're good.
-
@Mauritio Great, thanks.
-
Great info, thank you very much.
-
 T TexugoDoMel referenced this topic on
T TexugoDoMel referenced this topic on