Impaired ketogenesis in Leydig Cells drives testicular aging
-
A ketogenic diet has a number of negative issues. Using exogenous ketones, such as β-hydroxybutyric acid and acetoacetic acid, to restore more youthful levels of testosterone is an interesting idea.
Impaired ketogenesis in Leydig Cells drives testicular aging (2025)
Abstract
Testicular aging commonly leads to testosterone deficiency and impaired spermatogenesis, yet the underlying mechanisms remain elusive. Here, we show that Leydig cells are particularly vulnerable to aging processes in testis. Single-cell RNA sequencing identifies the expression of Hmgcs2, the gene encoding rate-limiting enzyme of ketogenesis, decreases significantly in Leydig cells from aged mice. Additionally, the concentrations of ketone bodies β-hydroxybutyric acid and acetoacetic acid in young testes are substantially higher than that in serum, but significantly diminish in aged testes. Silencing of Hmgcs2 in young Leydig cells drives cell senescence and accelerated testicular aging. Mechanistically, β-hydroxybutyric acid upregulates the expression of Foxo3a by facilitating histone acetylation, thereby mitigating Leydig cells senescence and promoting testosterone production. Consistently, enhanced ketogenesis by genetic manipulation or oral β-hydroxybutyric acid supplementation alleviates Leydig cells senescence and ameliorates testicular aging in aged mice. These findings highlight defective ketogenesis as a pivotal factor in testicular aging, suggesting potential therapeutic avenues for addressing age-related testicular dysfunction.
-
@DavidPS said in Impaired ketogenesis in Leydig Cells drives testicular aging:
oral β-hydroxybutyric acid supplementation
Not commonly sold in pure acid form due to formulation and stability issues...........What then?
-
@Wabi-sabi said in Impaired ketogenesis in Leydig Cells drives testicular aging:
Not commonly sold in pure acid form due to formulation and stability issues...........What then?
You can get them as salts of potassium, magnesium (or sodium and calcium). They need to be taken continuously throughout the day, though, as otherwise most will simply end up in urine if >3-4grams at once.
Also, hydroxybutyric acids increase secretory action in the GI system. Not good when diarrhea. Good for those with constipation.
We've had sourcing discussion in the context of HDACi recently. -
@CrumblingCookie - The mineral salts are the way the supplement industry is going. They seem to dissolve them to make a drink. I had not thought about slowly sipping the drink during the day. But it makes perfect sense that it is best not to consume man-made ketones at a rate higher than the body would product them.
-
@Wabi-sabi - Here a source that I found at Amazon.

-
@DavidPS
When buying it's worth to note that only D-beta-hydroxybutyrate can be sensibly used by the body. That goBHB brand doesn't say whether their product is racemic or not. Better confirm beforehand. -
@CrumblingCookie - Thanks that is good to know that as well.
I currently have no plans to consume ketones.
-
@DavidPS can this explain why some people have low t after long carb fasting aka high fat low carb diets?
-
@William-Shat said in Impaired ketogenesis in Leydig Cells drives testicular aging:
@DavidPS can this explain why some people have low t after long carb fasting aka high fat low carb diets?
I am not part of the keto community and I have not heard about this phenomenon before. So I am just an outsider who tries to eat a 'balanced diet' (rather than an unbalanced ketogenic diet).
For me the study was about restoring "Impaired ketogenesis in
Leydig Cells drives testicularaging". Are all of those "some people" aged?The sex steroids' are made from cholesterol. By way of analogy, I think of the sex steroids' as being part of a 'leaky pipeline system' that feeds into the production other upstream hormones subsystems before it feeds into the testosterone part. I have seen several schematic diagrams of the "pipeline'. Here is a diagram that suits my purposes.
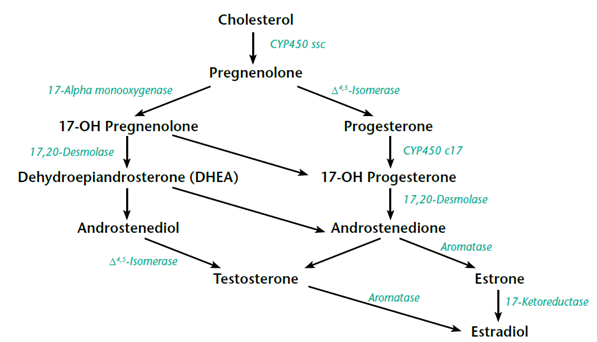
Cholesterol is at the head of the pipeline. I am wondering if those "some people" reduced their dietary cholesterol to the point where very little of it is left to trickle into testosterone production.
-
@DavidPS
I've thought along the same lines but struggled to fit those concepts coherently together.expression of Hmgcs2, the gene encoding rate-limiting enzyme of ketogenesis, decreases significantly in Leydig cells from aged mice. Additionally, the concentrations of ketone bodies β-hydroxybutyric acid and acetoacetic acid in young testes are substantially higher than that in serum, but significantly diminish in aged testes. Silencing of Hmgcs2 in young Leydig cells drives cell senescence and accelerated testicular aging.
So what I'll do is shower you and the others with my open questions:
First in line for me is the question: Why is (testicular) Hmgcs2 being downregulated in aging?
Obviously feeding BHB as the end product of ketogenesis increased testosterone. Therefore the underlying mechanism can't be about sex hormone substrate availability (cholesterol). Rather, it's about a decline in ketogenesis, i.e. a tissue energy depletion: "suppression of ketogenesis impairs steroidogenic function of LCs"
Is it locally or systemically/predominantly hepatically?
--> The study tells us it's locally, because testicular tissue concentrations are higher than serum and "BHB andAcAc concentrations in serum were comparable between the young
and the aged group (Fig. 2k, l)."
"Remarkably, the concentration of ketone bodies in the testes were more than tenfold higher than that in serum (Fig. 2i–l), implying ketone bodies might have testis-specific functions"Is there a decline in peripheral fatty acid circulation as a reason for declining tissue ketogenesis?
-
In contrast to ketone bodies, free fatty acids don't really cross the blood--brain-barrier. Do they significantly cross the blood-testis-barrier?
If it's not about fatty acids as a ketogenesis substrate, is it about peripheral protein circulation levels?
If it's about neither upstream substrates, is there perhaps a tissue-specific decrease of utilization to Acetyl-CoA? Is the HMGGCS2-decreasing cause founded in a lack of carnitine or a lack of CoA?
-
Will supplementation of carnitine or stimulation of CoA then attain similar results to exogenously supplying BHB for that matter?
--> The authors have followed this strain of thought and injected AAV vectors to deliver and overexpress the Hmgcs2 gene, demonstrating "that Hmgcs2 overexpression increased the levels of H3K9ac and FOXO3a in aged testicular tissue, similar to the levels in young testes"
--> So it's not immediately about any substrate levels but HMGCS2 activity itself.Does this lead back to epigenetic downregulation of bodily functions, in this case HMGCS2, and the concepts of DNMTi and HDACi?
--> Clearly there's a positive association between acetylation of H3K9 and HMGSC2 expression in rats in that HMGSC2 induced higher H3K9ac.
Is this reciprocal, i.e. would H3K9ac also induce HMGCS2?
Or does H3K9ac come about by increased concentrations of the products of HMGCS2, like BHB, and the cellular energy abundance provided by it?
--> The authors followed this and found: "Consistent with in vitro results, Western blot analysis revealed that BHB increased the levels of H3K9ac and FOXO3a in aged testicular tissue, similar to the levels observed in young testes (Fig. 7d, e)."
Is this then a course of gradual decline, wherein a decrease of testicular ketone energy in turn decreases its own synthesis? Just as the observation that more BHB increases its own synthesis? I.e. a positive / negative feedback loop?
Would, therefore, a one time course of exogenous BHB or induced Hmgcs2 overexpression "reset" testicular ketogenesis back to a youthful level, wherefrom it may once again gradually decline?
--> Well, the authors fed exogenous BHB in addition to the control diet to 18-months old rats for a duration of 2 months. Those were then 20-months old rats. Unfortunately, no subgroup to follow up on wrt to their physical activity and testicular findings has been kept and reported on beyond that.
"Last, we validated the changes in FOXO3a-related inflammation genes through quantitative RT-PCR analysis, revealing that inflammation-related genes were significantly upregulated in aged mice, and Hmgcs2 overexpression reduced the expression of these genes (Supplementary Fig. 9b–e).
Overall, these data suggest that enhancing ketogenesis is sufficient to alleviate LCs senescence and testicular aging in aged mice." -