Although I wouldn't decline some vorinostat aka SAHA aka suberoylanilide hydroxamic acid or romidepsin I'll go for the non-pharmaceuticals first:
• I am about to get the whole range of available butyrates; Na-But, Ca-But, Mg-But, K-BHB! And hopefully those will be as effective and pervasive into tissues as phenylbutyrate (without the nitrogen/glutamine/BCAA-depletion of the latter) or even better at an equivalent daily dosing between 10-20grs. The BHBs are and add-on for bOHbutyrylation, of the histone site H3K9, predominantly.
The different HDAC enzymes all preferably target different sites of the lysine chains.
• Choosing between sulforaphan (SFN) or erucin or isothiocyanates I'm going for EGCG to inhibit DNMT activity because the latter, in comparison, has the extra benefit of shuttling zinc across membranes and binding free iron instead of "merely" upregulating ferritin and intracellular metallothionine deposits. That action against free iron should prevent ferroptosis in healthy cells and increase intracellular TET proteins (which oxidize DNA methylation).
• Furthermore, maintain GSH (against hypermethylation) with extra NAC and
• maintain sufficient zinc, but not in excess!? Yet enough to competitively displace an overly abundant ratio of free iron and copper?*.
• As well as sufficient B2 and folate which are necessary for binding to a FAD‐dependent histone demethylase named lysine‐specific demethylase‐1 (LSD1). Folate is a necessary scavenger of formaldehyde which is being created by demethylization and therefore a coenzyme for demethylization enzymes as DMGDH, SDH in the liver.
• I'll avoid niacinamide because of its inhibition of PGC-1α through inhibition of SIRT1 (and also SIRT2) and rather use niacinic acid? And keep B5 at a sane, low amount to not overexpress CoA which is said to speed up the reaction rates of HDAC1 and HDAC2?
Would that be a solid approach?
Other HDACi:
• Btw butein looks good, too, but it also being a very potent tyrosine kinase inhibitor (decreases mitogenesis, cell division and cell proteins) sort of predestinates it for use in cancers with/by overactive TK activity rather than for non-anticancerous use, I guess.
• Puerarin looks interesting as it stimulates AMPK, PGC1α, Nrf2.
Increases autophagy, reduces Apoptose (PI3K/Akt activation = GSK3beta inhibition).
Inhibits mTOR, TNFα, NF-κB, JNK, Cox2, HDAC1 and HDAC3
Regulates PPARy.
• Apigenin ?! Inhibition of DNMT and HDAC.
• Decursin, which is a coumarin-derivative from Angelica plants. Predominantly DNMTi (looks for for synergy with a HDACi).
Orphan drugs or still in pharmaceutical trials:
• Panobinostat (cancelled in US, available in EU for >€4100/6 tablets)
• (Mocetinostat aka MGCD0103, inhibits mainly HDAC1, plus 2/3/11)
• (Entinostat aka MS-275 (patented), inhibits class I HDAC1 and HDAC3 with IC50 of 0.51 μM and 1.7 μM, respectively.)
• (Belinostat aka PXD101, only i.v.)
• Vorinostat aka SAHA aka suberoylanilide hydroxamic acid,
• Romidepsin, HDAC1,2 + HDAC4, 6 (weaker)
• Valproic acid
• Biotin: HDACi. Yet IME in a very specialized way because it's exacerbating when not in combination with a broad-range base.
• Vitamin E (which forms exactly?): HDACi. Yet IME in a very specialized way because it's exacerbating when not in combination with a broad-range base.
• Possibly alkanes like:
Terpenes such as Phellandren, α-Terpinen, Limonen, Undecan
- Resveratrol increases SIRT1. It's reported to inhibit DNMT1 and DNMT3b. It's a dirty, double-edged substance, imo, with a wide range of opposing effects. I don't like it much. It's kind of like eating super tasty icecream that has laxatives mixed into it.
Background info bits:
• The traditional DNA hypomethylating agents (HMAs) such as decitabine (DAC) and azacytidine (AZA) are super toxic and destructive.
• "The DNA methyltransferase (DNMT) family is comprised of the catalytically active DNMT1, DNMT3A, and DNMT3B, which methylate cytosines within CpG dinucleotides, as well as DNMT3L, which lacks catalytic activity and serves as a regulatory factor. Using S-adenosyl-l-methionine (SAM) as the methyl donor, DNMT3A and −3B establish the DNA methylation pattern de novo while DNMT1 is primarily responsible for propagation of this methylation pattern to daughter strands following DNA replication. Thus, DNMT1 preferentially binds to and methylates DNA containing a hemi-methylated CpG dinucleotide."
• On DNMT and HDAC interrelations: !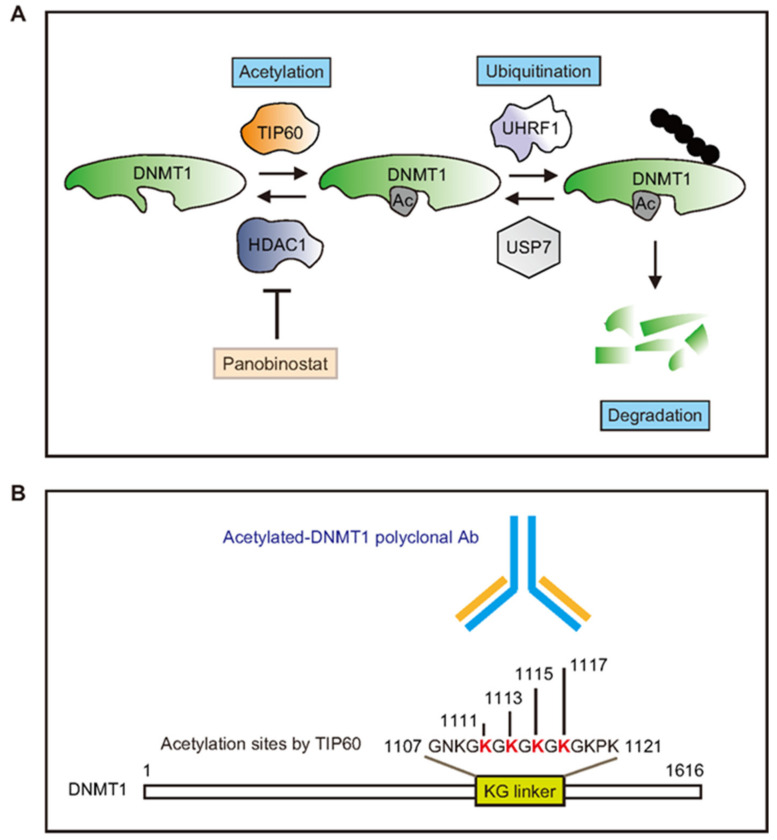
-> DNMT1 is acetylated by TIP60 promoting its degradation. TIP60 regulates DNMT1 acetylation, while histone deacetylase 1 (HDAC1) controls DNMT1 deacetylation.
• GDNF, BDNF etc are all influenced by inhibiting HDAC.
• Here's a bit on the decisive short-term benefits of proper vitamin A; Food for thought for everyone who feels acute benefits from Retinyls:
"retinol/retinoic acid (vitamin A) and ascorbate (vitamin C) synergistically diminish DNA methylation levels and in doing so enhance the generation of naïve pluripotent stem cells.
This is achieved by two complementary mechanisms. Retinol increases cellular levels of TET proteins (which oxidize DNA methylation), whereas ascorbate affords them greater activity by reducing cellular Fe3+ to Fe2+" [10.1073/pnas.1608679113, 2016]
Some papers:
• Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy, 2018
• Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation, 2020
• *: Therein, wrt zinc the HDAC familiy classification: 
• And from here the same with descriptions of the respective HDAC enzymes' main targets:
• Metabolism, HDACs, and HDAC Inhibitors: A Systems Biology Perspective, 2021
• Function and mechanism of histone beta-hydroxybutyrylation in health and disease, 2022
@CrumblingCookie said in Random, interesting studies:
@cs3000 said:
something interesting,
when thyroid receptors aren't bound by the hormone / agonist they block DNA transcription. using HDAC. HDAC inhibition is 1 way to help the people who have receptor mutation / resistance, (sort of)
https://academic.oup.com/hmg/article/23/10/2651/614693#10263757
https://scholars.mssm.edu/en/publications/histone-deacetylase-inhibition-reduces-hypothyroidism-induced-neu
So basically people can get some of the gene effects from T3 activation if its lacking, without the t3 , by hdac inhibition . not full effects but some
Bam! So the working mechanism of T3 is not directly cellular stimulation, but inhibition of the inhibition of cell metabolism?
Therefore, vice versa, a practically hypothyroid state would be mimicked by overmethylation of the CpG sites on the DNA (blocking the transcription of genes) or by deacetylated/dephosphorylated/demethylated/de-beta-hydroxybutyrylated sites of the lysine chains of the histones (compressing the histones which wrap the DNA strands which prevents DNA access)?
To supplement thyroid hormones would work in such circumstances but be a kind of force-feeding, rawhiding override and circumvention of the actual underlying culprits?
But using other inhibitors of HDAC or DNMT could then be actually better and closer to the original cause and also effectively act like thyroid hormones?
I used to think of HDACis only as some very beneficial class of substances in a vague context of cancer (even though even in that they are very restricted).
Now they appear much more crucial in all kinds of diseases and chronic impairments.
If I were casually being offered some pure quality HDAC inhibitors I would gladly take them and run a treatment course with them.
@alfredoolivas said in Random, interesting studies:
CrumblingCookie HDAC inhibition also increases expression of thyroid receptor itself.
@Mauritio said in Random, interesting studies:
@CrumblingCookie Good point .
Well, sodium butyrate is widely available ...