Anabolic Sensitivity in Healthy, Lean, Older Men
-
Lean and healthy old men (~age 70) do not show anabolic resistance to resistance exercise and amino acids.
More evidence that much of aging is actually just letting yourself go and becoming overfat and insulin resistant.
Staying lean and fit fights aging
-
@DavidPS said in Anabolic Sensitivity in Healthy, Lean, Older Men:
Staying lean and fit fights aging
Hi,
just to make a up, and contribute ...
Nothing to do with insulin insensitivity but as overfat, insulin insensitivity and inflammation are members of the same trilogy, we could add one more point: lack of potassium, preventing adequate cleansing. I explain (context needed):Oxidized Cholesterol, Macrophages and Potassium
Potassium to dampen high tension and prevent AVC. Atherosclerosis begins with macrophage engulfment of oxidized LDL C-foam cells (process to clean / get rid of Ox-LDL.
=> 2 g K+ more makes a difference (3 g as middle take). So 3 + 2 = 5 000 mg.Excerpt (translated)
Macrophages are cells that infiltrate tissues and have a cleaning role. They are notably responsible for purifying LDL cholesterol (the wrongly-called “bad” cholesterol). But when this cholesterol is oxidized, macrophages store it in the arterial walls and become the raw material for atherosclerotic plaque. They are then called “foam cells”. By preventing macrophages to adhere to arterial walls, potassium could slow down cardiovascular disease.
Translated from the book “Potassium: Mode d’Emploi”. Dr. Philippe VEROLI.
Chapitre 5 – Prévenir et Traiter les maladies Cardio-Vasculaires.
Sources and References- The role of macrophage ion channels in the progression of atherosclerosis
&3. Ion channels in macrophages
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1225178/full
Potassium channels are crucial regulators of macrophage membrane potential and ion homeostasis. - Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines
https://pubmed.ncbi.nlm.nih.gov/8558596/
We have identified an outwardly rectifying K+ channel that appeared to be involved in cytokine and adherence-mediated macrophage activation, and in the maintenance of cytoskeletal integrity and cell shape. - Inverse relationship between potassium intake and coronary artery disease in the cholesterol-fed rabbit
American Journal of Hypertension, 1999, https://doi.org/10.1016/S0895-7061(99)00039-4 - Atherosclerosis begins with macrophage engulfment of oxidized LDL C-foam cells (process to clean / get rid of Ox-LDL.
Researchgate (access denied with VPN)
- The role of macrophage ion channels in the progression of atherosclerosis
-
@LucH - Thanks for the information on potassium. I purposefully have high amount of potassium in my diet for other reasons.
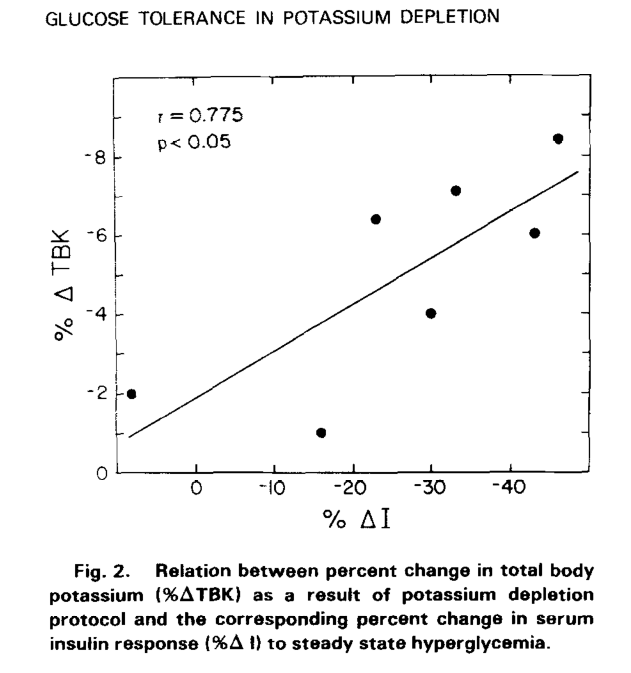
Effect of experimental potassium deficiency on glucose and insulin metabolism (1980)
Disturbances in glucose and insulin metabolism frequently accompany a variety of clinical states associated with potassium deficiency. The exact role of the potassium deficit and the mechanism of its effect are in doubt. The glucose-clamp technique was therefore employed to study glucose and insulin metabolism in 7 normal young male subjects before and after induction of potassium depletion. The clamp technique places the blood glucose concentration under the investigator's control. Under the conditions of steady state hyperglycemia (125 mg/dl above basal for 2 hr) it provides quantification of (1) pancreatic beta cell sensitivity to glucose (plasma insulin response), (2) glucose tolerance (glucose metabolized), and (3) tissue sensitivity to insulin (glucose metabolized/insulin response). Potassium deficiency was induced during a 7–8 day period of a weight-maintaining diet containing 40 meq potassium and at least 150 g carbohydrate, along with the administration of 60 g Na polystyrene sulfonate daily. Paired analysis showed a significant decline in the amount of glucose metabolized from pre- to postdepletion (−27.4 ± 4.5%, p < 0.01). This decline in carbohydrate tolerance was associated with a significant decrease in plasma insulin response to sustained hyperglycemia (−26% ± 6.9%, p < 0.02). Potassium depletion had no effect on tissue sensitivity to insulin (+1.7 ± 7.8%). The degree of potassium depletion as estimated by change in total body 40K ranged from 1.0% to 8.4% and correlated with the decrease in insulin response (r = 0.78, p < 0.05). This study demonstrates that potassium depletion causes glucose intolerance, which is associated with impaired insulin secretion.