Contextualizing D-lactate
-
Selected points:
- L-LDH is cytosolic, whereas D-LDH is mitochondrial, but distributed close to the surface
- D-LDH: highest concentrations in liver and kidneys (3× the tissue activity of the liver), and lowest in brain
- D-lactate is oxidized to pyruvate at low concentrations, but (unlike L-lactate) it tends to be excreted intact in urine as its plasma concentration rises
- Normal, low concentration: 0.01 mmol/L
- Recovery starts to saturate at 1 mmol/L and it's almost completely saturated at 5 mmol/L
- The tubules of kidneys are permeable to unionized (or non-charged) molecules (such as lactic acid)
- Acidosis unionizes part of the circulating D-lactate to D-lactic acid, promoting its passive recovery from filtrate when it would otherwise be lost, decreasing the clearance rate
- Baking soda should counteract the effect, serving to ionize it and promote excretion, regulating D-lactate level as drugs
- In contrast, fast intestinal fermentation of carbohydrates can increase the acidity, changing part of D-lactate to D-lactic acid
- D-lactic acid when crossing compartments drags an additional proton along (beyond those incorporated), which contributes to acidemia and a vicious cycle
- D-lactate can be formed endogenously from methylglyoxal (primary source)
- The average consumption of propylene glycol (as food additive) is about 2.5 g/day and half of it may yield D-lactate after metabolism
- If you're on Windows, it's possible to open a symbols panel with 'Win key + period', and there must be something similar in superior OSs
- The clearance rate of D-lactate is 50% of L-lactate in normal subjects
↳ L-lactate: 1.89 L/min to 0.72 L/min
↳ D-lactate: 0.71 L/min to 0.43 L/min (going from plasma 0.115 mmol/L to 5.24 mmol/L) - D-lactate concentrations found in sauerkraut: 80 mmol/l
- D-lactate concentrations found in unstrained yogurts: 29 to 122 mmol/l
- Lactulose given at 160 g/day resulted in fecal concentrations of 43 mmol/L and ⅓ being D-lactate
Their estimate in short bowel syndrome:
Carbohydrates:
-
277 g/day ingested
↳ 17%: 47 g/day excreted
↳ 83%: 230 g/day retained -
230 g/day retained
↳ 40%: 92 g/day absorbed
↳ 60%: 138 g/day fermented to D-lactate
Molecular weight of monosaccharides:
180 g per 1 mol; or 1 g per 1/180 mol- 138 g/day fermented to D-lactate
↳ 138 g/day × 1/180 mol = 0.77 mol D-lactate
As in the other thread, the yield is two pyruvates or lactates per toasted monosaccharide:
- 0.77 mol × 2 = 1.54 mol or 1540 mmol D-lactate
Around 500 mmol in each of 3 meals a day, with simulated peaks of 6 or 4 mmol/L, depending on faster or slower absorptions. As the amount of carbohydrates increases, it gets worse.
But the effect was subtle when healthy people were stuffed with yogurt. Not all yogurts are rich in D-lactate, yet they picked one that was, proving 40 mmol or 75 mmol D-lactate per meal, from 800-1600 g of unstrained yogurt that they had to ingest.
In these conditions, the plasma peak after the consumption of what corresponds to an entire large container was 0.2 mmol/L.
↱ [40] Postprandial plasma D-lactate concentrations after yogurt ingestion
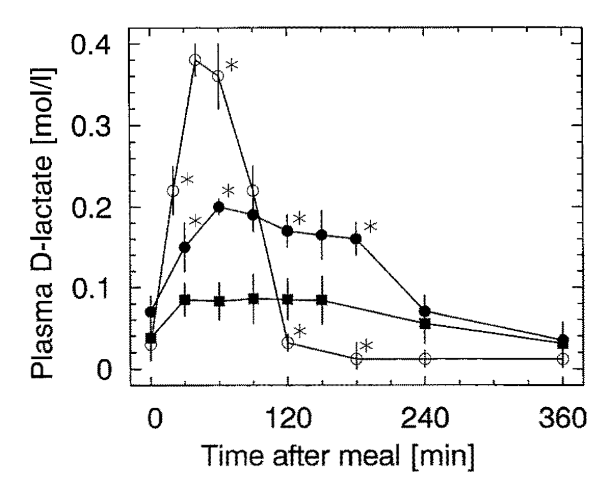
- ○ 1.1 mmol D-lactate/kg bw* -- as DL-lactate with water
- ⬤ 1.1 mmol D-lactate/kg bw* (7 g/victim) -- from yogurt
- ■ 0.6 mmol D-lactate/kg bw (4 g/victim) -- from yogurt
*Total absorption was similar, only smoothed out in the case of yogurt.
"Since only a minor fraction of intravenously administered D-lactate is excreted intact in the urine,[10]** the bulk of D-lactate elimination from the plasma requires oxidation to pyruvate.** Following the description of D-lactic acidosis, biochemists searched for a D-lactate oxidizing enzyme with the characteristics of L-LDH (ie, cytoplasmic location, reversible reaction with pyruvate, NAD+ cofactor, etc.). When no such enzyme was identified, it was thought that humans lack D-LDH; rather D-lactate catabolism was attributed to a multipurpose mitochondrial enzyme termed D-alpha-carboxy acid dehydrogenase.[11] Recently, however, it was demonstrated that humans have a gene that molecular biologists term D-LDH because of its ability to code for a protein that catalyzes the oxidation of D-lactate to pyruvate in fungi and prokaryotes.[12,13] When humans with inactivating mutations of this gene were observed to have elevated plasma D lactate concentrations, it was concluded that the human wild-type gene coded for a protein with D-lactate oxidizing ability. Thus, it appears that the mitochondrial D-LDH of the molecular biologist is the D-alpha carboxy acid dehydrogenase of the biochemist – the long-standing controversy of whether humans have D-LDH was a semantic problem. It is now generally accepted that humans have a D-LDH that is structurally and functionally an entirely different enzyme than is L-LDH. D-LDH, unlike L-LDH, is located on the inner mitochondrial membrane and catalyzes an irreversible reaction to pyruvate. The activity of this enzyme depends on transporters to deliver D-lactate to the inner surface of the mitochondrial membrane, and defects in these transporters could lead to reduced D-lactate oxidation to pyruvate.[14] D-LDH activity is widely distributed in various tissues with the highest concentrations in the liver and kidney and the lowest concentrations in brain. Since the only means of elimination of D- and L-lactate is via conversion to pyruvate followed by oxidation of pyruvate, the elimination rates of the two optical isomers are dependent upon the relative activities of D-LDH and L-LDH. Older literature reported that elimination of D-lactate was much slower than that of L-lactate. However, multiple recent studies have uniformly demonstrated that the elimination rate of D-lactate in normal subjects is about 50% of that of L-lactate."
"While L-lactate elimination is almost entirely via catabolism, D-lactate is both catabolized and excreted intact in the urine. Lactate is freely filtered by the glomerulus, and since it is more than 99.9% in the anion form (at a pH of 7), tubular reabsorption requires a carrier mediated system. During an intravenous infusion of D-lactate, Oh et al[10] quantitated the renal excretion of D- and L-lactate in normal control subjects as a function of the plasma D-lactate concentration. Tubular reabsorption of D-lactate starts to saturate at a plasma concentration of 1 mM, with near-complete saturation at a concentration of 5 mM. Thus, urinary D-lactate excretion is negligible at low concentration and increases to about 80% of the creatinine clearance at 6 mM.
While urinary excretion has been assumed to play a major role in the elimination of D-lactate at the high plasma concentrations observed in D-lactic acidosis, this clearly was not the case in patients with D-lactic acidosis for whom D-lactate clearance can be estimated.[8,15,16] As shown in Table 1, urinary D-lactate clearance when blood lactate was >6 mM was <7 mL/min, less than 10% of the 80 mL/min predicted for normal subjects. Thus, urinary excretion plays a negligible role in D-lactate elimination in patients with D-lactate acidosis."
"Since renal function was near normal in most of the subjects listed in Table 1, low urinary clearance cannot be attributed to renal failure. Rather, in these acidotic patients, a larger fraction of the tubular lactate will be in the unionized form in the acidic urine, allowing more rapid non-ionic diffusion from the tubules. At the urine pH of 5.0 observed in severe metabolic acidosis,[3] 6% of lactate will be unionized, compared to just 0.06% at pH 7.0. This hypothesis is supported by studies with salicylic acid.
- The cortical collecting duct permeability increased 6.5 fold for salicylic acid when urine pH decreased from 6.0 to 5.0,[4] and
- proximal tubule permeability increased 5.6 fold when pH decreased from 7.0 to 6.0.[5]
Since the pKa of salicylic acid is 3.0, less than that of lactic acid (3.8), the predicted increase in lactic acid permeability should be greater than that of salicylic acid. This non-ionic diffusion would not have been observed in Oh’s study[10] since lactate (not lactic acid) was infused, and the subjects would not have been acidotic."
So, don't inject yourself with D-lactate and let it coincide with acidosis because the tubules of kidneys are permeable to non-charged molecules (lactic acid). The toxin is recovered passively when it would otherwise be lost. Taking baking soda should help to regulate D-lactate level in acidosis as drugs, by promoting its elimination.
"Whole-body D-lactate clearance (catabolism plus renal excretion) has been directly assessed in four studies involving normal control subjects, summarized in Table 2. Two different techniques were used. Kuze et al[17] and Oh et al,[10] constantly infused D-lactate until a steady state was reached, at which point the rate of removal must equal the infusion rate, and clearance is determined from the steady-state concentration. Connor et al[18] constantly infused D-lactate for 20 minutes and then determined clearance using a non-compartment pharmacokinetic analysis of the plasma concentration curve. The clearance of D-lactate in these studies was surprisingly fast, ranging from 0.43 to 0.71 L/min/70 kg. The results in suggest that the clearance decreases as the plasma concentration rises, decreasing from 0.71 L/min at 0.115 to 0.43 L/min at 5.24 mM. For simple Michaelis-Menten kinetics, this would correspond to a Km of about 6 mM [when half of the capacity is reached], similar to that observed in vitro measurements of rat liver metabolism[9]."
"Extensive study of L-lactate clearance in healthy controls has yielded values ranging from 0.72 L/min/70 kg to 1.89 L/min/70 kg. Thus, D-lactate clearance is about 50% of that of L-lactate. Of interest, data from two studies[2,19] in which D-lactate was infused into cattle allowed us to estimate the normal D-lactate clearance of these animals. Our calculated clearances were 40 and 31 mL/min/70 kg, only about 5 −7% of that of humans, which may explain the propensity of cattle to develop D-lactic acidosis."
"Based on in vitro tissue assays, the kidney and liver are the main sites of D-lactate metabolism, with the kidney having about 3 times the activity per gm than does the liver.[20] Fine[21] infused D-lactate into the portal circulation of dogs and directly measured the hepatic extraction from the portal vein – hepatic vein concentration difference. They found a relatively small extraction of about 14%, which, when extrapolated to humans (hepatic blood flow of about 1.5 L/min), corresponds to a clearance of about 210 mL/min, about one-third of the total human D-lactate clearance. Presumably, the other two-thirds of the catabolism is via the kidney."
"Normally, lactate is found in very low concentration (<2 mM) in crap. The well-accepted scenario for over-production of D-lactate in the colon of SBS subjects is that massive delivery of readily fermentable carbohydrate to the colonic bacteria results in rapid organic acid production and a concomitant fall in luminal pH. The acidic pH inhibits acetate and butyrate forming bacteria and selects for lactic acid bacteria,[6] and lactic acid accumulates in the feces. Mayeur et al[28] showed that lactobacilli routinely were the predominant fecal organism in 16 SBS subjects; however, 8 of these subjects had normal (<2 mM) fecal lactate concentrations, ie, the activity of the non-lactate flora was sufficient to maintain normal fecal lactate concentrations. The other 8 subjects were lactate “accumulators”, with fecal concentrations ranging from 20 mM to 165 mM. Of importance, D-lactate comprised greater than 90% of the lactate in two subjects, each of whom had a past history compatible with D-lactic acidosis."
"The quantity of carbohydrate that must be delivered to the colon to induce a predominantly lactic acid producing colonic flora was investigated by Hove and Mortenson[29] who measured the fecal lactate concentrations of a small group of healthy subjects fed varying doses of lactulose, a totally non-absorbable disaccharide. At the maximal dosage of 160 g/day, total fecal lactate concentration rose to 43 mM, of which about one-third was D-lactate."
"The quantity of lactate produced in and absorbed from the colon of SBS patients is not known. What is known is that 16 SBS subjects (without D-lactic acidosis) ingested an average of 277 g/day of carbohydrate and excreted about 17% of this carbohydrate in some form per rectum (measured as the difference between total fecal calories minus fat + protein calories).[28] Thus, an average of 230 g/day of carbohydrate “disappeared” from the intestine via either monosaccharide absorption from the small bowel or organic acid absorption from the colon. Assuming that small bowel absorption of carbohydrate in SBS is similar to the 40% absorption measured for fat and protein, 60% of 230 g/day of carbohydrate or 138 g/day of carbohydrate was absorbed from the colon, presumably almost all in the form of organic acids. If D-lactate were the predominant fecal organic acid (as shown to be likely in Mayeur’s study), the absorption rate of D-lactate theoretically might approach 1550 mmol per day, the value used for the predictions shown in Figure 3."
"Given the mechanism of action of MCT, the absorption rate of lactate should be a function of the hydrogen ion gradient across the epithelial membrane. Such was found to be the case in in vivo studies in the sheep intestine[31] where the absorption rate of D-lactate increased by 6 fold, as the luminal pH was reduced from 6.3 to 4.3, a range of values observed in the feces of SBS subjects. Thus, the very acid luminal pH resulting from the rapid fermentation of malabsorbed sugars in SBS facilitates the absorption of D-lactate at a rate sufficient to produce the syndrome. As will be discussed, the movement of a proton with D-lactate during MCT facilitated transport is the likely cause of the acidosis of the D-lactic acidosis syndrome."
"Methylglyoxal, a highly toxic intermediate in the metabolism of a variety of compounds, may be catabolized to D-lactate.[32] This pathway is thought to be the major, non-gut, endogenous source of D-lactate. Assuming this pathway to be the sole source of blood D-lactate in healthy people, the rate this pathway normally delivers D-lactate to the circulation can be calculated from D-lactate clearance and the plasma D-lactate concentration. Normal plasma lactate concentration has been reported to range from 0.006 mM (high performance liquid chromatography) to 0.25 mM. For illustrative purposes, a clearance of 600 mL/min/70 kg and a steady-state plasma D-lactate concentration of 0.010 mM yields a D-lactate elimination rate = input rate of about 0.006 mmol/min/70 kg, or about 8.6 mmol/day."
"The output of D-lactate from the methylglyoxal pathway can increase in several pathological conditions. An association between diabetic ketoacidosis and plasma D-lactate elevations was initially observed in cats[33] and more recently in humans,[34] who had plasma D-lactate concentrations averaging 3.82 mM (15 and 8 times greater, respectively, than controls and non-ketotic diabetic subjects). The highly significant, positive correlation between the D-lactate concentration and anion gap suggested that D-lactic acidosis was contributing to the diabetic acidosis. The source of the D-lactate in ketoacidosis is thought to be via increased input of ketone bodies into the methylglyoxal pathway."
"Propylene glycol is a widely used solvent that exists as a racemic mixture of the D- and L- enantiomer. The D-fraction is metabolized via the methylglyoxal pathway to D-lactate, and markedly increased plasma D-lactate concentrations have been observed in cats fed large doses of propylene glycol (> 1 g/kg).[35] Similarly, a patient who mistakenly ingested a large quantity of propylene glycol was reported to have D-lactate concentrations as high as 110 mM/l (a seemingly impossible concentration given the anion gap of only 27 mM).[36] The solubilizing/preservative/anti-caking properties of propylene glycol has led to its incorporation into a wide a variety of foods. The average 70 kg US subject ingests about 2.4 g (31 mmol) of propylene glycol per day.[37] About 45% of this propylene glycol is excreted unchanged in urine, and about 50% of the remainder appears to be metabolized to D-lactate. Thus, about 8 mmol per day (25% of the normal ingestion) might be converted to D-lactate. Although probably coincidental, the daily normal intake of propylene glycol could roughly account for the small quantity of D-lactate, about 9 mmol, previously calculated to enter the plasma each day of healthy subjects."
"Propylene glycol is present in very high concentrations (up to 80% by volume) in some intravenous medications including lorazepam, phenobarbital, phenytoin and nitroglycerine. Prolonged infusions of these drugs have been well documented to produce lactic acidosis although the exact roles played by D- and L-lactate have not been well defined.[38]"
"The ingestion of D-lactate containing foods or infusion of D-lactate intravenous fluids provide a source of D-lactate. Lactic acid bacteria play a role in virtually all food fermentation processes, and a variable fraction of the resulting lactate is D-lactate. Yoghurt and sauerkraut have received the most study. Two bacteria, Lactobacillus bulgaricus and Streptococcus thermophilus, are commonly added to milk to bring about fermentation to yoghurt. The relative proportions of each lactate isomer varies with the bacteria employed. In one study of different yoghurts, D-lactate concentrations ranged from 29 to 122 mmol/l [2.5 to 11 g/L].[39] DeVrese and Barth[40] measured plasma D-lactate concentrations after ingestion of 74 mmol/70 kg of D-lactate (800–1600 g of yoghurt). The plasma concentration peaked at only 0.20 mM at one hour (
Figure 4). Calculation of the fractional absorption of D-lactate utilizing the PBPK modeling procedure indicated that 38.2 mmol (52% of the ingested D-lactate) reached the peripheral circulation (Figure 4), with absorption rate constants of TT = 21.6 and TA = 120 minutes, similar to the slow absorption rate assumed in Figures 3, 6 and 7.""Defective D-lactate elimination could reflect a baseline loss of D-LDH activity due to renal or hepatic tissue injury. Alternatively, there could be inhibitors of D-LDH activity that function at baseline or are operative only during an episode of D-lactic acidosis. An example of the former is oxalate, a potent in vitro inhibitor of D-lactate oxidation; eg, an oxalate concentration of 15 µM halved the rate of catabolism of 3 mM D-lactate.[45] Normal plasma oxalate concentrations are <2 uM, and there appear to be no measurements of plasma oxalate concentration in SBS subjects. However, hyperoxaluria, calcium oxalate stones, and oxalate nephropathy, complications of SBS, are indicative of elevated plasma oxalate concentration. Patients with end-stage renal disease have plasma oxalate concentrations of >50 uM,[46] and thus would be expected to have very slow D-lactate clearance if oxalate were an in vivo inhibitor of D-LDH. While patients with renal failure being treated with chronic peritoneal dialysis had baseline plasma D-lactate concentrations of about 0.07 mM (seven times normal), intraperitoneally infused D-lactate was metabolized at a normal rate, a result that does not support a role for oxalate in the pathogenesis of D-lactic acidosis."
"It also has been proposed that acidosis associated with elevated plasma D-lactate concentrations inhibit D-LDH. This concept derives from an in vitro study of Tubbs and Greville[11] showing that D-lactate oxidation has a broad pH optimum around 8.0, and that this activity falls off rapidly at pH 7.4. The pH optimum of 8.0 makes “sense” given the location of D-LDH in the mitochondrial matrix which maintains a very alkaline pH.[47] It seems unlikely that the plasma pH decrement observed in D-lactic acidosis would have an appreciable effect on D-LDH activity via an alteration of intra-mitochondrial pH. The effect of acidosis on human D-lactate catabolism has not been investigated – all measurements of lactate catabolism have utilized an IV infusion of D-lactate, which has an alkalizing effect. However, there are two D-lactate infusion studies in calves, one that used acidified D-lactate such that the plasma pH fell to 7.24[2] and a second study using D-lactate in a neutral solution where no reduction in pH occurred.[21] Our calculated D-lactate clearance for the study with acidosis was 40 mL/min/70 kg and with no acidosis, 31 mL/min/70 kg, a result that does not support the concept that acidosis inhibits D-lactate catabolism."
"In contrast to yoghurt, sauerkraut is fermented by lactobacilli naturally attached to the cabbage. Measurements made on the sauerkraut supernatant showed that D-LDH activity far exceeded that of L-LDH and that D-lactate was the predominant form of lactate (concentrations of roughly 80 mmol/l [7 g/L]).[41] As discussed with yoghurt, ingestion of sauerkraut, even at doses of 1 liter, would result in a trivial increase in blood lactate."
"Another source of exogenous D-lactate is lactate containing intravenous solutions that usually contain 28 meq/l of racemic lactate, ie, 14 meq/l of each isomer. Our modeling studies indicate that infusion of this quantity of D-lactate should result in only minor increases in plasma D-lactate, and Kuze et al[17] found that subjects infused with D-lactate at a rate of 14 mmol/hour (equivalent to 1 liter of Ringers-lactate/hour) for 3.5 hour rapidly developed a steady-state plasma lactate concentration of only about 0.3 mM. Similarly, the use of a lactate containing solution for peritoneal dialysis showed peak plasma D-lactate concentrations of only about 0.25 mM that returned to normal before the next instillation of dialysate.[42]"
"The belief that defective D-lactate catabolism plays a major role in the pathogenesis of D-lactic acidosis has led to a good deal of speculation as to factors that might reduce D-LDH activity of SBS subjects. Defective D-lactate elimination could reflect a baseline loss of D-LDH activity due to renal or hepatic tissue injury. Alternatively, there could be inhibitors of D-LDH activity that function at baseline or are operative only during an episode of D-lactic acidosis. An example of the former is oxalate, a potent in vitro inhibitor of D-lactate oxidation; eg, an oxalate concentration of 15 µM halved the rate of catabolism of 3 mM D-lactate.[45] Normal plasma oxalate concentrations are <2 uM, and there appear to be no measurements of plasma oxalate concentration in SBS subjects. However, hyperoxaluria, calcium oxalate stones, and oxalate nephropathy, complications of SBS, are indicative of elevated plasma oxalate concentration. Patients with end-stage renal disease have plasma oxalate concentrations of >50 uM,[46] and thus would be expected to have very slow D-lactate clearance if oxalate were an in vivo inhibitor of D-LDH. While patients with renal failure being treated with chronic peritoneal dialysis had baseline plasma D-lactate concentrations of about 0.07 mM (seven times normal), intraperitoneally infused D-lactate was metabolized at a normal rate, a result that does not support a role for oxalate in the pathogenesis of D-lactic acidosis.[42]"
"It also has been proposed that acidosis associated with elevated plasma D-lactate concentrations inhibit D-LDH. This concept derives from an in vitro study of Tubbs and Greville[11] showing that D-lactate oxidation has a broad pH optimum around 8.0, and that this activity falls off rapidly at pH 7.4. The pH optimum of 8.0 makes “sense” given the location of D-LDH in the mitochondrial matrix which maintains a very alkaline pH.[47] It seems unlikely that the plasma pH decrement observed in D-lactic acidosis would have an appreciable effect on D-LDH activity via an alteration of intra-mitochondrial pH. The effect of acidosis on human D-lactate catabolism has not been investigated – all measurements of lactate catabolism have utilized an IV infusion of D-lactate, which has an alkalizing effect. However, there are two D-lactate infusion studies in calves, one that used acidified D-lactate such that the plasma pH fell to 7.24[2] and a second study using D-lactate in a neutral solution where no reduction in pH occurred.[21] Our calculated D-lactate clearance for the study with acidosis was 40 mL/min/70 kg and with no acidosis, 31 mL/min/70 kg, a result that does not support the concept that acidosis inhibits D-lactate catabolism."
D-Lactic Acidosis: An Underrecognized Complication of Short Bowel Syndrome
Alteration of the colonic microbiota plays a major role in the production of D-lactate. Short bowel syndrome leads to an increased load of undigested carbohydrates (including simple sugars) in the colon. As a result, the amount of organic acid produced exceeds the amount that can be metabolized by healthy individuals. This leads to an accumulation of organic acids, including SCFA and lactate, resulting in a more acidic environment than normal. Interestingly, the lower pH favors the growth of bacteria responsible for producing D- and L-lactate as they are acid-resistant and this leads to a further decrease in the pH thus generating a vicious cycle. These bacteria include Lactobacillus fermenti and L. acidophilus amongst a few others [25,26]. As mentioned previously, the primary enzyme responsible for metabolizing D-lactate in humans is D-2-HDH, which is inhibited in the acidic environment. Caldarini et al. [27] demonstrated this in an in vitro study performed on stool samples of a child. Thus, SBS leads to initiation and propagation of D-lactic acid formation.
Other conditions that can mimic SBS in producing the complications of D-la include inflammatory disease of the small bowel, especially Crohn’s disease, antibiotics, and even probiotics that can alter the existing flora of the colon.
Patients with SBS have fat malabsorption and this can lead to calcium soap formation in the gut with free oxalate being absorbed with the risk of renal stones. Patients should stay well hydrated to keep up with the losses. Also oxalate can inhibit D-2-HDH, and hence oxalate intake should be limited. Small amounts of calcium supplementation (up to 1 g/day) may be beneficial. This will also increase the pH in the bowel, which could decrease D-lactate production as shown by Caldarini et al. [27].
D-Lactic Acidosis in Humans: Review of Update
"D-lactate is normally produced by the fermentative organisms of the gastrointestinal tract, mainly by lactobacilli and bifidobacteria. Under normal condition, lactate is not produced in acid-base imbalance because it is converted by other microbes to acetate and fatty acids. The major benefit of these organic acids in the gastrointestinal tract is to provide a fuel for oxidative metabolism and ion pumping for mucosal cells of the colon11). The colon is protected from large influxes of carbohydrate, being regulated by gastric emptying and effective small intestinal digestion and absorption."
-
@Amazoniac I think this can be why making one's own kefir is fun and beneficial. The flora of the gut seems to be crucial for the absorption of certain foods and nutrients and potentiation of processes in the body as it seems lactobacillus and bifidobacterium improve thyroid usage, testosterone retention and neurotransmitters production.
Perhaps one of the reasons a 'diet' doesn't work for some or doesn't work to the extent desired is because the gut ecosystem hasn't been properly established. It's like raising a baby, snake or opening a deli there needs to be an understanding of responsibility.
Step 1. Add beneficial bacteria
Step 2. Feed beneficial bacteria
Step 3. Be mindful of overgrown and the bacteria spending all your money
Step 4. Taking bicarb soda before bed can be helpful
Step 5. Build out a diet that keeps this flora thriving whilst meeting all nutritional needs.
Step 6. Maybe the first step should be culling the bad bacteria however some beneficial bacteria can do this for you
Step 7. ProfitIt does make sense that our bodies are equipped to deal with fermented end products as history would deem it necessary to preserve food in times of need. I like to think of it as a healthy gut is helpful the same way healthy people are, the healthy gut wants you to thrive so it can also thrive so it speeds up metabolism so you can acquire more food for it as an unhealthy gut wants to slow you down to survive as long as it can.
-
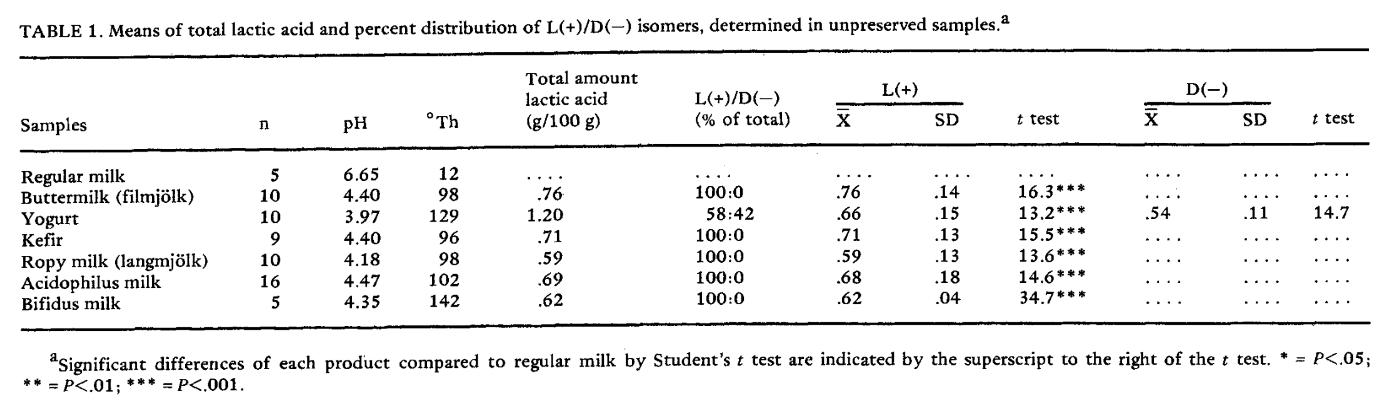
Effect of Fermentation on L(+) and D(−) Lactic Acid in Milk
A Stand-Alone Synbiotic Treatment for the Prevention of D-Lactic Acidosis in Short Bowel Syndrome
"To eliminate D-lactate–producing bacteria completely, thorough intestinal decontamination was carried out. Specifically, metronidazole (Flagyl, Shionogi & Co, Ltd, Osaka, Japan) 500 mg/d and kanamycin 2000 mg/d were administered for 5 days under fasting conditions. Polymyxin B (Polymyxin B Sulfate, Pfizer Japan Inc, Tokyo, Japan) 500 × 103 U/d and vancomycin (Vancomycin Hydrochloride Powder, Lilly, Kobe, Japan) 1000 mg/d were administered over the subsequent 5 days. After the use of antibiotics, a purgative (Niflec, Ajinomoto Pharmaceuticals Co, Ltd, Tokyo, Japan) was used."
"After these decontaminations, although two Gram-stained samples of stool showed that microorganisms like Candida constituted nearly all of the intestinal flora, the fecal concentration of D-lactate in 2 samples of stool remained detectable (4.5 and 4.4 mmol/L, respectively), which indicated impossibility of eliminating D-lactate–producing bacteria completely using this methodology (
Table 1). We understood that it was more important to suppress the overgrowth of D-lactate–producing bacteria than to eliminate them.""Overgrowth suppression was approached by starting synbiotics, specifically B breve Yakult (prepared by Yakult Co, Ltd, Tokyo, Japan) 3.0 g/d and L casei Shirota (Biolactis Powder, Yakult Co, Ltd, Tokyo, Japan) 3.0 g/d as probiotics, and galacto-oligosaccharide 8.4 g/d as a prebiotic."
"We used as probiotics B breve Yakult and L casei Shirota, which are known to produce only L-lactate. D-lactic acidosis was successfully controlled by this treatment for 3 years (i.e., the patient was free of recurrence and had a good quality of life, such as having unlimited oral intake). There were no reports that described successful management of D-lactic acidosis continuously for such a long period."
"Bustos et al11 reported that fecal concentrations of D-lactate tended to be high in patients with SBS even when serum D-lactate levels were undetectable. The authors pointed out that the development of D-lactic acidosis requires not only overproduction of D-lactate in the colon, but also other factors, such as absorption or impaired D-lactic acid metabolism. Synbiotics have been shown to produce a large amount of short-chain fatty acids by correcting intestinal flora.5 These fatty acids promote proliferation of the intestinal epithelium and stimulate intestinal motility.12 Through active bowel movements, D-lactate produced by colonic fermentation can be expelled with feces prior to excessive absorption in the colon. In the present case, although fecal concentration of D-lactate was high (24.8 mmol/L), serum D-lactate levels (2.4 mmol/L) maintained a level lower than the threshold of symptom onset (>2.5 mmol/L), which allowed the patient to remain asymptomatic. Actually, the patient had frequent defecation and persistent loose stools after starting synbiotic treatment. Therefore, it was likely that synbiotics increased intestinal peristalsis, resulting in prevention of excessive absorption of D-lactic acid."
-
@Amazoniac I assume you are what you eat does indeed make sense as long as there isn't an overgrowth. Throughout history I'd imagine when times are plentiful and fruits are easily obtained that the microbiome created from ingesting these foods would create a flora to push metabolism and neurotransmitters like dopamine to go explore and find more carbs and then in times of survival when more grains needed to be consumed that the microbiome would tend towards slowing down and making it through. Our ancestors likely roll their eyes at our 'discovery' of the relevance of the microbiome when it probably should be instinctual. I wondering if bathing in kefir is going too far Dont kefir the reaper