Zonulin and its Consequences
-
Tight Junction(TJ) proteins are crucial components of the cellular structures that form barriers between cells in epithelial and endothelial tissues. These proteins help maintain cell polarity and control the passage of molecules and ions through the space between cells, known as the paracellular pathway. Dysfunction of tight junctions is casually referred to as leaky gut.
Leaky Gut and Autoimmune Diseases*
A century ago, TJs were conceptualized as a secreted extracellular cement forming an absolute and unregulated barrier within the paracellular space [22]. Biological studies of the past several decades have shown that TJs are dynamic structures subjected to structural changes that dictate their functional status under a variety of developmental scenarios.
The discovery of zonula occludens toxin (Zot), an enterotoxin elaborated by Vibrio cholerae that reversibly opens TJ[23], increased our understanding of the intricate mechanisms that regulate the intestinal epithelial paracellular pathway and led to the discovery of its eukaryotic counterpart zonulin [24, 25].
This pathway appears to be involved in several functions, including TJ regulation responsible for the movement of fluid, macromolecules, and leukocytes between the bloodstream and the intestinal lumen and vice versa.
GI symptoms in diabetes mellitus have been generally ascribed to altered intestinal motility secondary to autonomic neuropathy [35].However, other studies suggest that an increased permeability of intestinal TJ is responsible for both the onset of the disease and the GI symptoms that these patients often experience [36]. This hypothesis is supported by a study performed on a spontaneously diabetic animal model [37]. The authors of this study showed an increased permeability of the small intestine of Bio Breeding Diabetes Prone (BBDP)/Wor diabetic-prone rats that precedes at least a month the onset of diabetes [...] We confirmed these data by reporting in the same rat model that zonulin-dependent increase in intestinal permeability precedes the onset of T1D by 2–3 weeks [38]. Oral administration of the zonulin inhibitor, AT1001 (now called Larazotide acetate), to BBDP rats blocked autoantibody formation and zonulin-induced increases in intestinal permeability, so reducing the incidence of diabetes [38]. These studies suggest that the zonulin dependent loss of intestinal barrier function is one of the initial steps in the pathogenesis of T1D in the BBDP animal model of the disease. The involvement of zonulin in T1D pathogenesis was corroborated by our studies in humans showing that ~50% of T1D patients has elevated serum zonulin levels that correlated with increased intestinal permeability [39].
In clinically asymptomatic Crohn’s disease patients, increased intestinal epithelial permeability precedes clinical relapse by as much as 1 year, suggesting that a permeability defect is an early event in disease exacerbation
Before you get too excited, read this sensible article positing that serum zonulin isnt a reliable biomarker to test for leaky gut: The Clinical Utility of Zonulin Testing
here's a tidbit:
The basic idea is that with zonulin, a fairly large (47 kDa) protein produced in the gut, should not be able to pass into the bloodstream under normal conditions. Thus, its recovery in plasma or serum likely reflects the degree of intestinal permeability. While the theory makes sense, any good biomarker worth its predictive value will also have the following characteristics:
- Sensitivity
- Specificity
- Robustness
- Accuracy
- Reproducibility
In the next several sections, I’ll discuss why zonulin doesn’t hold up to these standards.
Note his final section, "Is It Even Worth Testing for Leaky Gut?" is ill-informed, asserting:
intestinal permeability itself is almost always caused by something further upstream
this is incorrect, as the original article demonstrates:
1. Elevated serum zonulin precedes symptoms
2. Zonulin inhibitors are protectiveHowever there are still a handful of reasonable contentions with looking only at zonulin, and some actionable alternatives provided.
Hopefully this is instructive to those who feel they may be suffering from leaky gut/autoimmune conditions and are looking for specific mechanisms of action and practical interventions.
*full text available in the usual places
-
@stag said in Zonulin and its Consequences:
Zonulin inhibitors are protective
I don't see it so.
Zonulin and lectins
I don’t see zonulin as a protective inhibitor in presence of deficient tight junctions.
For me zonulin is secreted in presence of excess lectins and gliadin (agglutinin family) to avoid aggregation with L-glutamine from the membranes. Zonulin acts as a garde-barrière, telling the body to let the toxins get away. Zonulin tells the tight junctions to stay open …A FASANO · 2011 · This zonulin-driven opening of the paracellular pathway may represent a defensive mechanism which flushes out microorganisms so contributing to the innate immune system...
https://www.esi.academy/wp-content/uploads/18-Zonulin-and-Its-Regulation.pdf
Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function. -
@LucH if you eat gluten foods but take aspirin before the meal the effect is suppressed .
-
@Bling5 said in Zonulin and its Consequences:
@LucH if you eat gluten foods but take aspirin before the meal the effect is suppressed .
It would be useful if ...
Agglutin attacks the membranes. If salicylic acid neutralizes / combines with lectins and gliadin, it would be fine. Neutralized before combining with L-glutamine from the membranes..
Do you think so / have your read sth going on this way? -
Deduction of what I’ve read.
Salicylic acid (SA = Aspirin) contributes to a local and systemic acquired resistance (SAR) in plants. SAR is thus a resistance mechanism against pathogen infection (micro-organisms) or invaders. SA is secreted and upregulated when needed to protect.
What is the probable role of salicylic acid in the defense responses of plants?
SA is considered a ubiquitous signaling molecule involved in conferring biotic and abiotic stress tolerance in plants by regulating the expression of critical genes and proteins of the stress defense pathway (Ding and Ding, 2020).
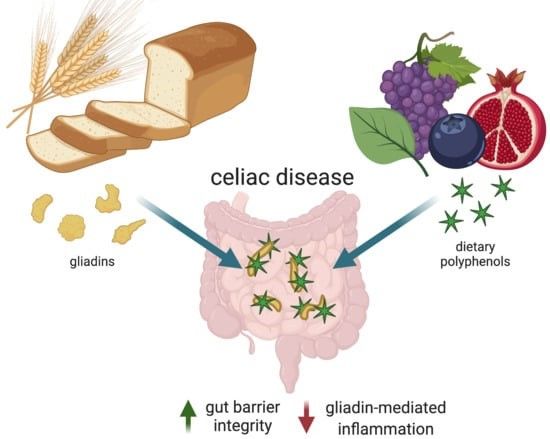
So, the defense system is upregulated and SA modulates the immune reaction but it doesn’t say if agglutinin molecules are precipitated / sequestrated like some ECGG from green tea could do it with gliadin from wheat.
Int J Mol Sci. 2021 Jan. doi: 10.3390/ijms22020595
Gliadin Sequestration as a Novel Therapy for Celiac Disease: A Prospective Application for Polyphenols. -
@LucH theres a study from like 1980 or something where a celiac person takes aspirin before meals and they dont get symptoms of gluten problems . when they dont take the aspirin they do
-
@Bling5 said in Zonulin and its Consequences:
theres a study from like 1980 or something where a celiac person takes aspirin before meals and they dont get symptoms of gluten problems . when they dont take the aspirin they do
Thanks for this precision. It means side-effects are downregulated: less immune reaction, thus lowering inflammation and digestive issues.
Note that it's probably a temporary effect if you don't change the way you manage. It calms down but it doesn't deal with the cause. The problem will soon upraise by another way.I'm searching for studies showing how lectins and gluten could be hydrolyzed or sequestrated.
but alpha-gliadin resists.
Recent studies suggest that preventing the digestion and absorption of gluten proteins by sequestering the protein from interaction with the gastrointestinal tract may be an effective, novel therapy for celiac disease.
https://www.mdpi.com/1422-0067/22/2/595
-
@LucH apparently wheat also messes with something to do with gamma linoleic acid conversion in diet. something peaters probably get little to none of due to pufa avoidance.
-
@LucH That's a very comprehensive paper, thanks for sharing!
I didn't mean that zonulin is a protective inhibitor, I was referencing that substances that inhibit zonulin (like Larazotide acetate) are protective against its deleterious effects (like type 1 diabetes / celiac), as elaborated on in the papers we have both posted.
@LucH said in Zonulin and its Consequences:
For me zonulin is secreted in presence of excess lectins and gliadin (agglutinin family) to avoid aggregation with L-glutamine from the membranes. Zonulin acts as a garde-barrière, telling the body to let the toxins get away. Zonulin tells the tight junctions to stay open …
Do you have more info on this? A search for lectins and leaky gut discloses this article:
Lectins: The Gluten-Lectin-Leaky Gut ConnectionSome lectins that we consume in everyday foods can bind to the sugars in the cell walls of the gut or in the blood. This can cause an immune response, leading to inflammation, intestinal damage, altered gut flora, malabsorption, decreased cellular repair, cellular death, and eventually disease.
These lectins bind to glycoproteins and glycolipids (sugar-coated proteins and fats) found on the surface of human and other animal cells. This binding allows for agglutination (clumping) and sometimes can produce an immune response. They can cause agglutination of blood cells and they can bind to the cells that line the small intestine.
This article also references Fasano, he seems to be a popular guy in zonulin world. The relevant reference regarding lectins is here (I think):
Dietary lectins are metabolic signals for the gut and modulate immune and hormone functionsA related paper is here:
Characteristics and consequences of interactions of lectins with the intestinal mucosaFollowing general Peat diet suggestions will have you avoiding most lectin-containing foods anyway, but there are two that stick out: dairy and nightshades. Someone could be getting most of their calories from milk and potatoes thinking theyre fine because its Peaty but may be driving intestinal permeability due to the lectin content.
The example given in the article of a noxious lectin is wheat germ agglutinin. I wonder how bad non-wheat derived lectins like those from potatoes and milk are.
If the mechanism is as described I also wonder if it may be advisable to keep dietary gluten and lectins to a minimum during L-glutamine supplementation, as that combination may provide ample reason for a zonulin trigger. This will be easy for wheat, likely also potatoes, but cutting milk may prove to be a challenge, if necessary. It also may be the case that the supraphysiological doses of glutamine generally used in supplementation may override any zonulin signaling caused by incidental dietary lectins. I'm not sure.