On Cellular Organization and Respiration
-
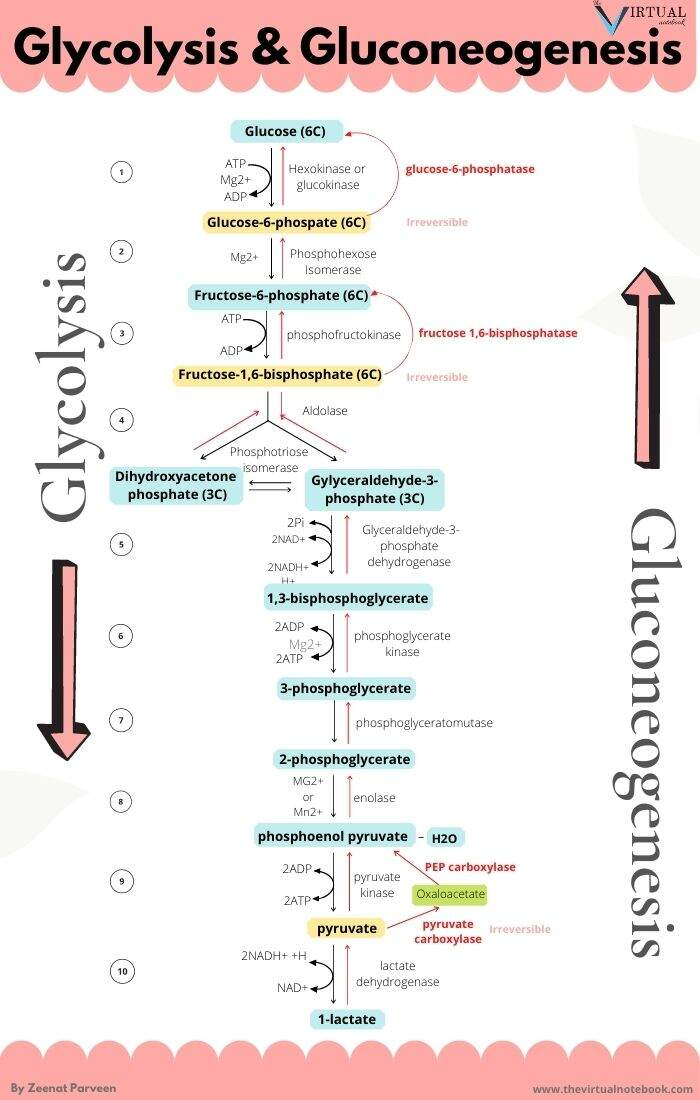
The metabolic steps responsible for the ATP difference in the breakdown and synthesis of dextructose:
Glycolysis (↓) Enzyme Gluconeogenesis (↑) –1 ATP HK N/A–1 ATP PFK N/A+2 ATP PGK –2 ATP +2 ATP PK N/AN/APEPCK –2 ATP (⇄ 2 GTP) N/APC –2 ATP Total Total +2 ATP (–4 ATP/↺) –6 ATP With the exception of PGK, these are the irreversible steps; the others not included are reversible.
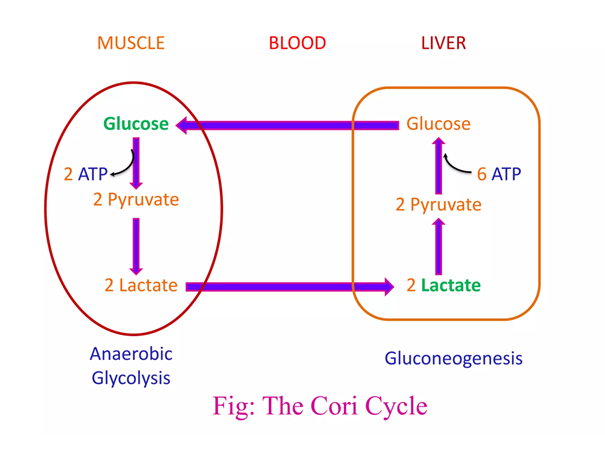
If forming and unforming lactate occurred in the same cell, it would have a neutral effect. However, for pyruvate to reappear elsewhere, NADH is consumed in the origin and NAD⁺ in the destination.
-
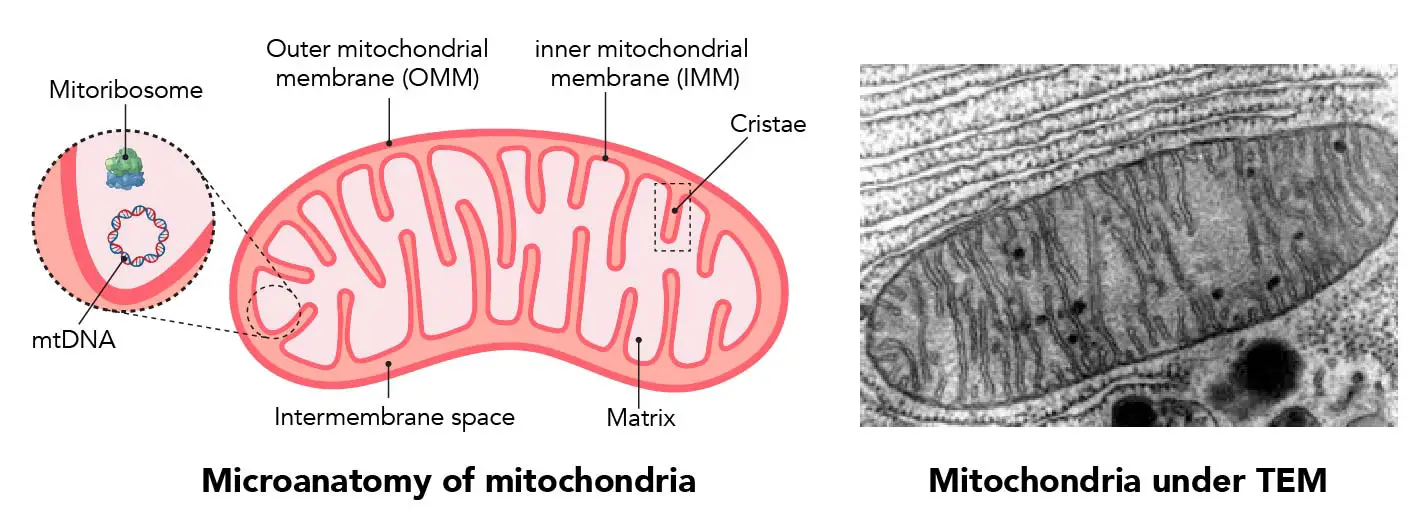
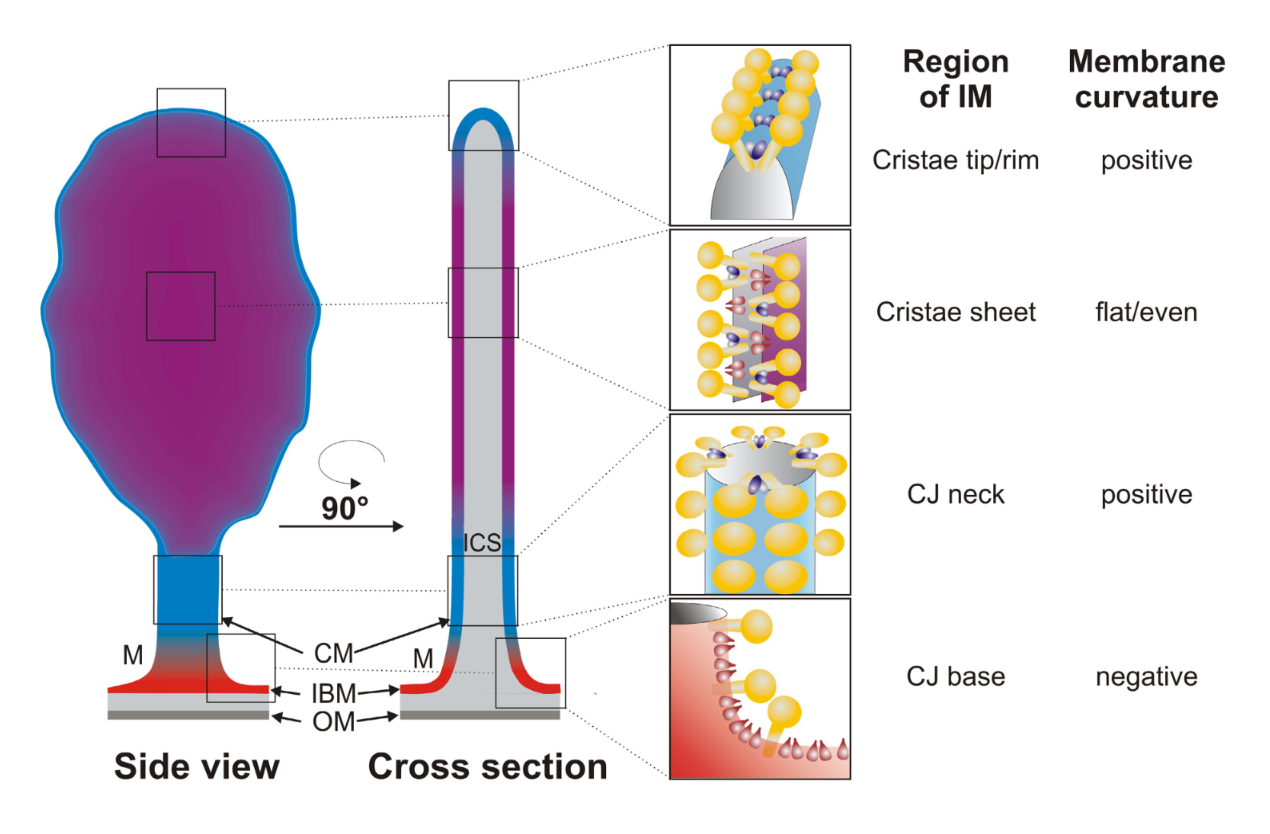
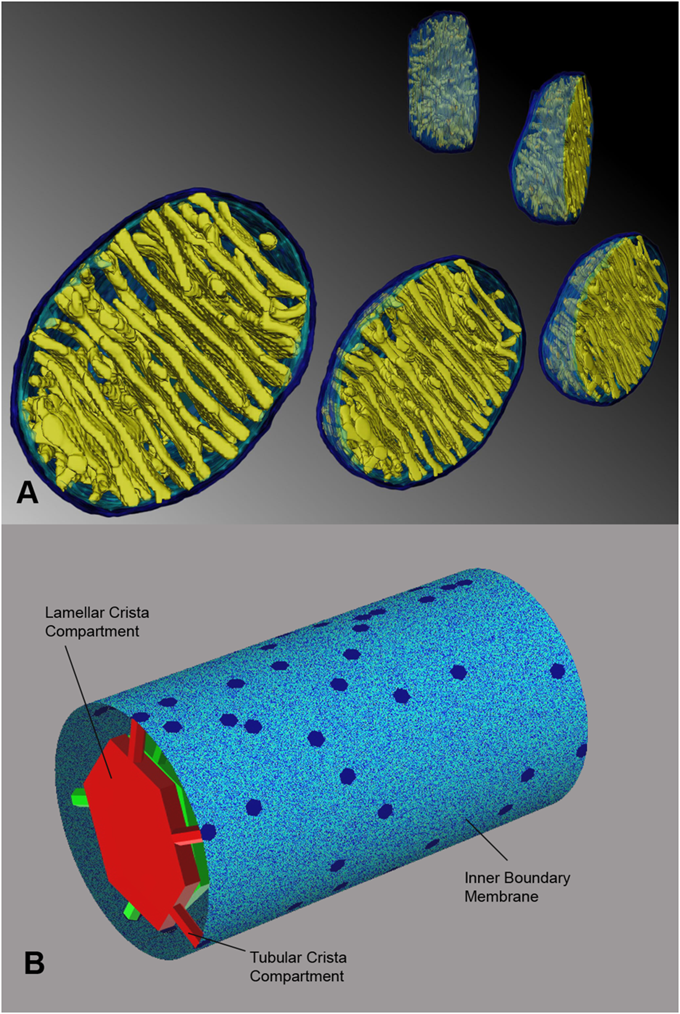
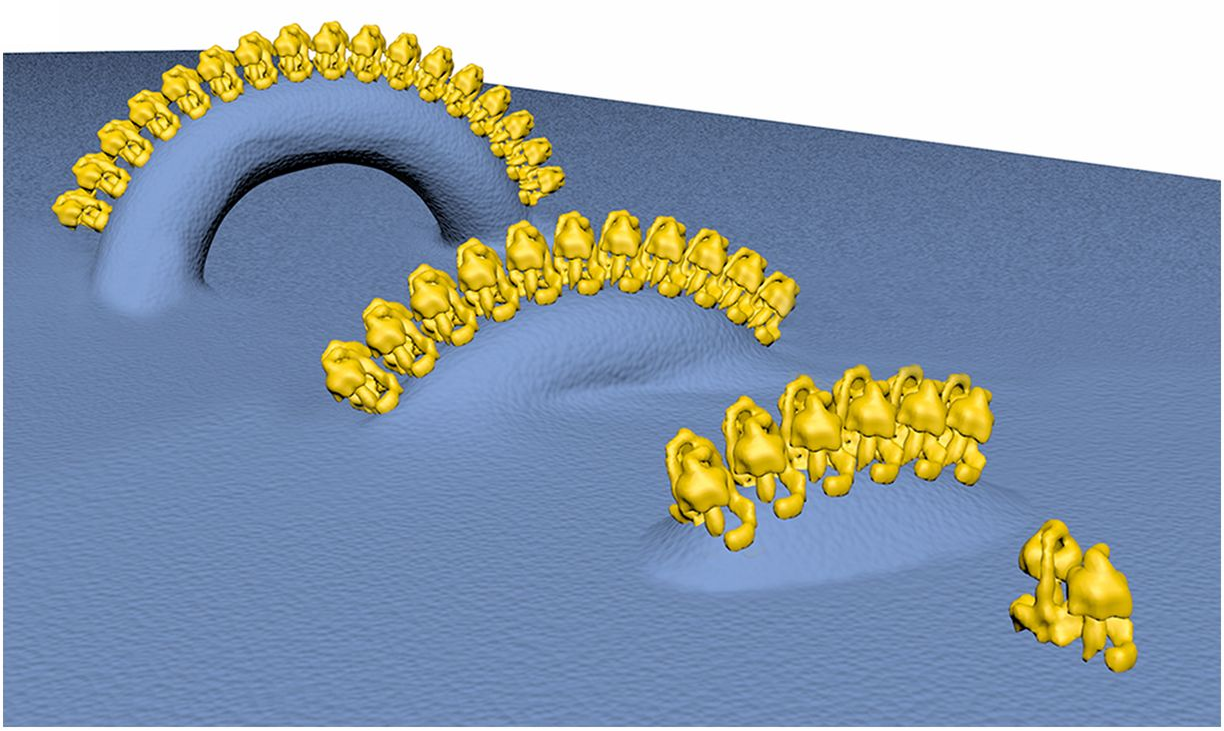
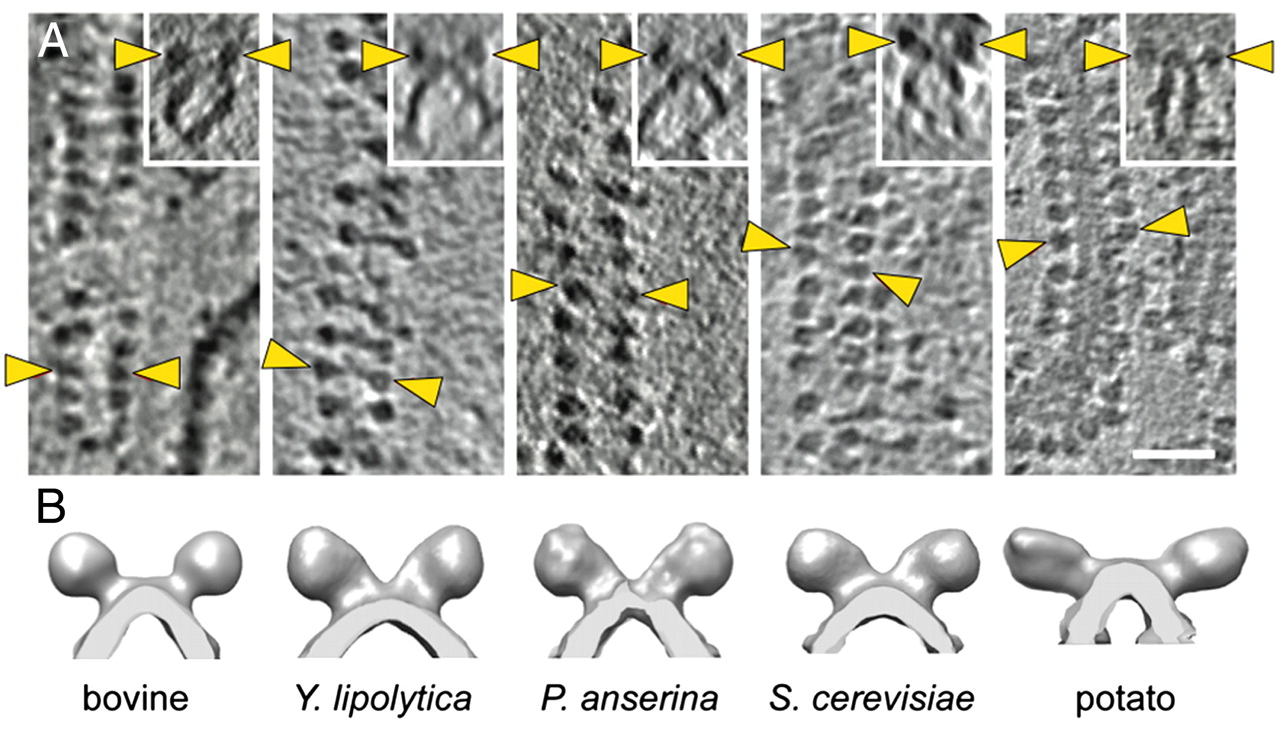
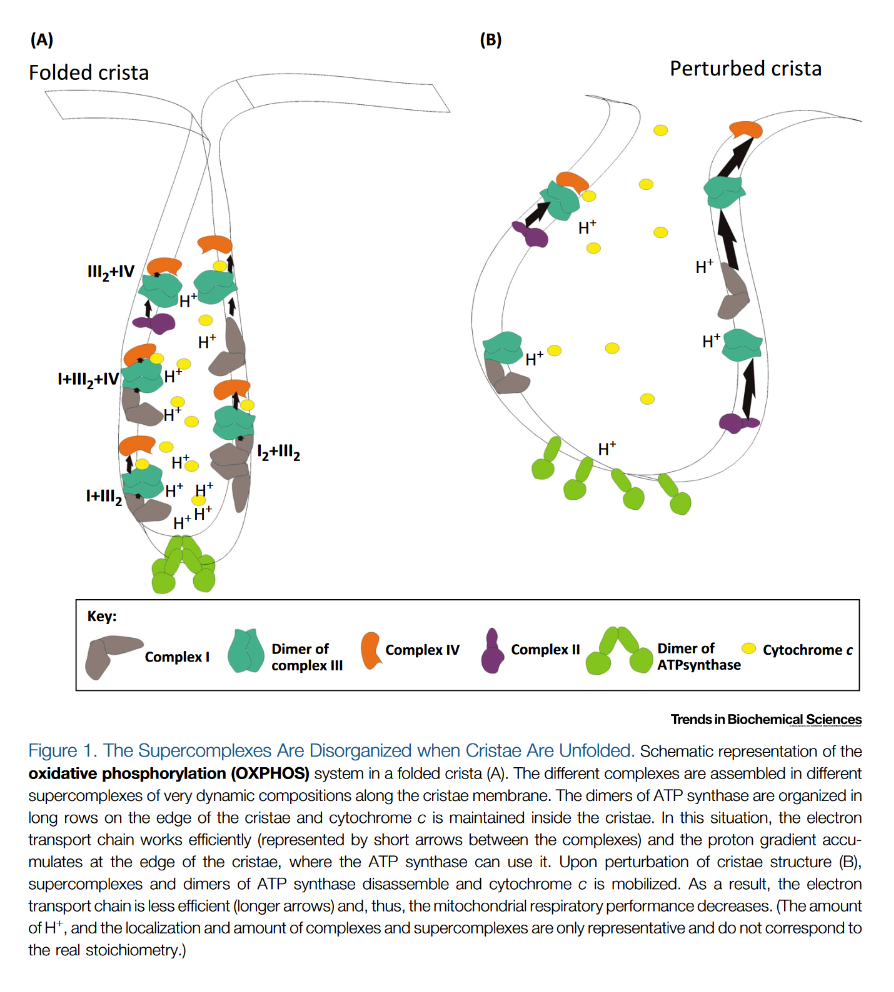
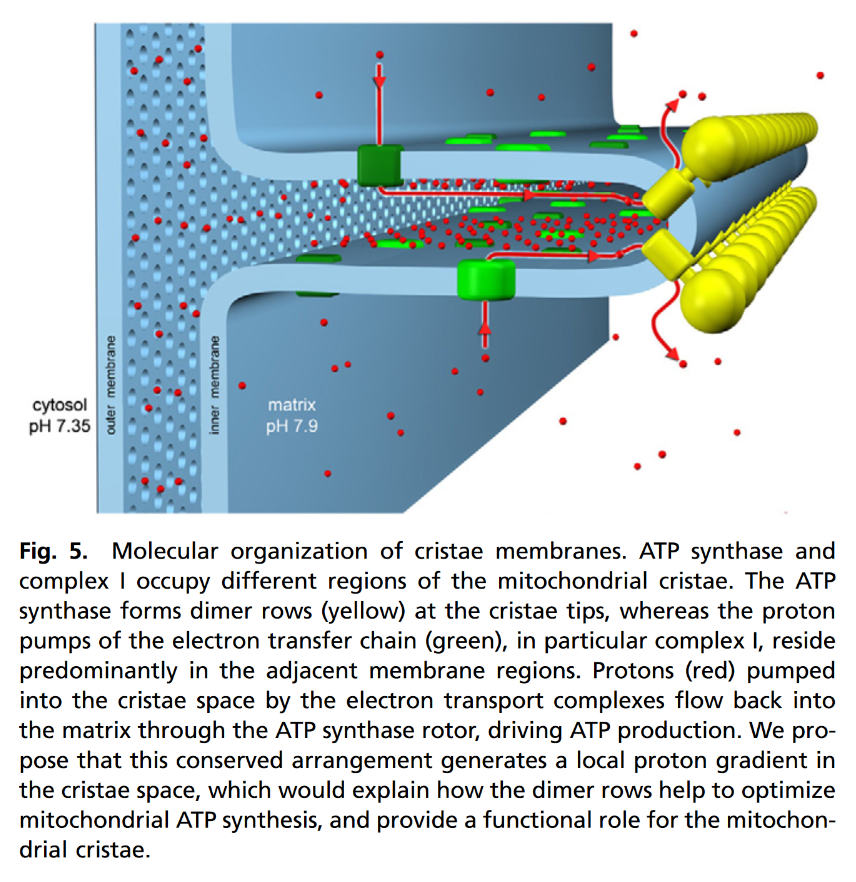
"The IMM consists of subcompartments called cristae and inner boundary membrane (IBM) (Palade, 1953). Cristae are invaginations protruding into the mitochondrial matrix, whereas the IBM runs parallel to the outer mitochondrial membrane (OMM). Cristae and IBM are connected via narrow tubular or slit‐like structures, known as crista junctions (CJs). In recent years, studies show that components of the electron transport chain (ETC) are confined to the lateral surfaces of the cristae rather than equally distributed along the IMM (Vogel et al, 2006; Wilkens et al, 2013). Moreover, dimers of F1F0 ATP Synthase assemble in rows along the edges of the cristae (Dudkina et al, 2005; Strauss et al, 2008; Davies et al, 2011). The CJs can be kept in a closed state by oligomers of the inner‐membrane dynamin‐like GTPase, OPA1 (Frezza et al, 2006; Pham et al, 2016), as well as components of the mitochondrial contact site and cristae organizing system (MICOS complex) (John et al, 2005; Rabl et al, 2009; Barrera et al, 2016; Glytsou et al, 2016)."
"Measurement of ΔΨm in individual cristae reveals that crista junctions provide electrical insulation and sustain polarization of individual mitochondrial cristae within a single mitochondrion even when neighbouring cristae are damaged.
- Cristae have higher ΔΨ compared to their adjoining inner mitochondrial membranes.
- Cristae are electrically insulated, allowing individual cristae within any given mitochondrion to have different membrane potentials.
- Cristae can remain polarized despite depolarization of neighbouring ones.
- Disruption of crista junctions impairs the electrical insulation of cristae, equilibrating their ΔΨ with those of inner mitochondrial membranes."
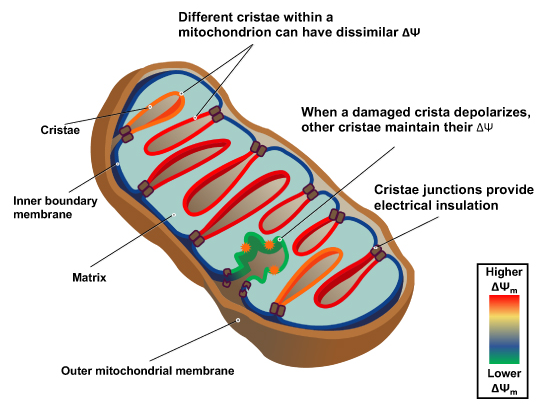
"This study raises interesting questions as to why mitochondria organize the ΔΨm in this way. One advantage, for example, could be related to the fact that ΔΨm constitutes the main energy available to drive protons through F1F0 ATP Synthase to produce ATP. As such, the localization of F1F0 ATP Synthase at the cristae rims appears to be advantageous in terms of proximity to the batteries. Another possible advantage could be compartmentalization of ΔΨm in each crista may serve as a safeguard mechanism restricting the impact of localized damage. In the case of the equipotential model, where the inner membrane of the entire mitochondrion represents a single capacitor, a breach in membrane integrity in one crista would cause a collapse in voltage in all cristae and compromise the function of the whole organelle. If, on the other hand, the IMM could maintain numerous, discrete electrochemical gradients, like a group of batteries, then failure of one or more would not invariably jeopardize the entire mitochondrion. This may be of particular relevance in cells harboring a highly interconnected mitochondrial network as opposed to cells with less elongated and/or branched mitochondria. Furthermore, the hetero‐potential model suggests that cristae with higher ΔΨCr‐IBM could compensate for cristae with impaired function."
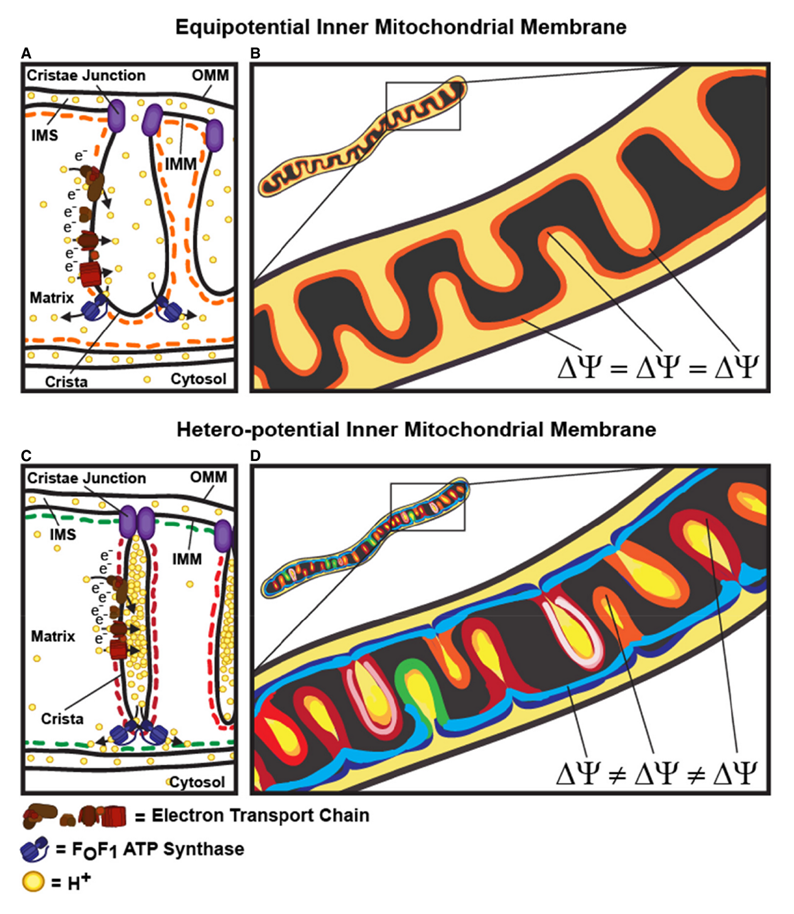
"Hyperpolarized and depolarized IMM potentials are associated with different states of respiration. While both uncoupling and an increased rate of ATP synthesis dissipate ΔΨm, a decrease in ATP synthesis may result in hyperpolarization and increased ROS production. The hetero‐potential model of the mitochondrion allows for different cristae to serve different functions. In this model, some cristae could be more dedicated to ATP synthesis, whereas neighboring cristae could play a role in ROS signaling. The hetero‐potential model further allows for the consideration that different cristae may engage in primarily complex II vs. complex I respiration, which are associated with different membrane potentials and could be driven by different fuels."
Protonic Capacitor: Elucidating the biological significance of mitochondrial cristae formation
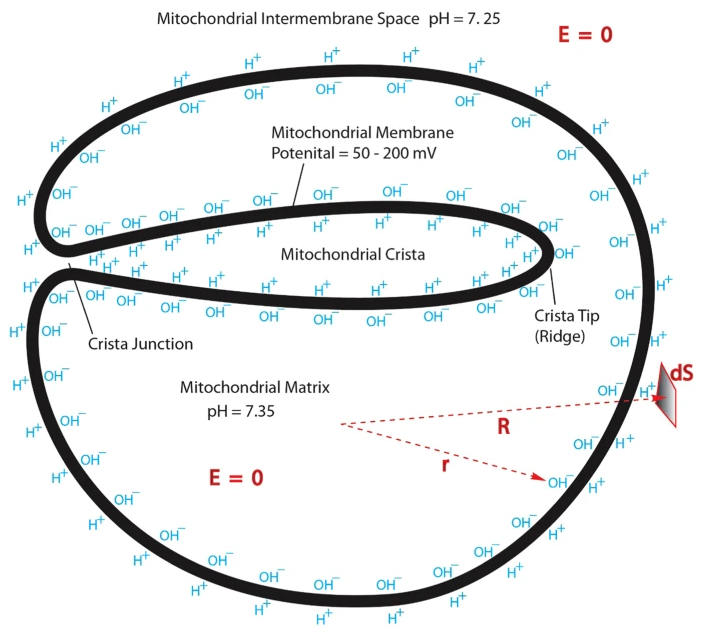
"[..]excess protons (positive charges) in an aqueous liquid on one side of a membrane will repel each other to become electrostatically localized along the membrane surface, attracting an equal number of excess hydroxyl anions (negative charges) to the other side of the membrane and thus resulting in a “protonic capacitor structure” (Fig. 2)."
Consequences of Folding the Mitochondrial Inner Membrane
"The greater the energy requirements of a cell, the more inner membrane surface area it contains. Because there are practical limits to the volume fraction that cells can reserve for mitochondria, crista packing is maximized where energy demand is greatest, e.g., in cardiomyocytes the surface area of the inner membrane is more than tenfold that of the outer membrane."
"Although internalizing the chemiosmotic membrane is essential for mass production of ATP, it creates a complex and potentially risky situation for the cell. In particular, conditions that swell the matrix will cause the inner membrane to unfold and, eventually, rupture the outer membrane. In fact, cells use this demolition mechanism when death is the intended outcome. For example, inner membrane “herniation” of the outer membrane is observed in late stages of programmed cell death (extrinsic apoptosis) in FAS-activated liver (
Figure 1). Crista contents, including cytochrome c, spill into the cytosol, resulting in irreversible loss of membrane potential and ATP production (Mootha et al., 2001). Matrix swelling in this case was attributed (Feldmann et al., 2000) to the mitochondrial permeability transition pore, MPTP, the opening of which can drive an osmotic influx of water sufficient to unfold the inner membrane and rupture the outer membrane (e.g., Rasola and Bernardi, 2011).""Extreme crista swelling is as perilous to the cell as uncontrolled matrix swelling, e.g., the total volume of a few fully expanded cristae in a single muscle mitochondrion easily exceeds the volume enclosed by the outer membrane. In fact, rupture of the outer membrane by crista (not matrix) swelling occurs in insect flight muscle as a prelude to apoptosis (Walker and Benzer, 2004). Clearly, the process of unfolding the inner membrane is as important to cell survival as generating the crista folds and likely is regulated as carefully."
"Although, at first glance, it seems risky to fold a large membrane within an outer membrane, rupture of which is fatal, this situation actually provides the cell an advantage. When mitochondria are suspended in hypo-osmotic media, outer membranes lyse at sucrose gradients tenfold greater than liposomes or mitochondrial inner membrane vesicles of similar size, typically 20–30 mM (Douce et al., 1972; Li et al., 1986). This dramatic protection against osmotic stress directly accrues from the outer membrane being osmotically inactive, i.e., very permeable to small solutes. The chemiosmotic inner membrane is the mitochondrial osmometer. Swelling of the matrix caused by osmotic influx of water compresses the cristae before significant pressure is applied to the outer membrane by outward expansion of the inner membrane. In effect, unfolding the inner membrane absorbs significant osmotic stress and delays irreversible damage to the mitochondria. Equally important, this indirect rupture mechanism provides the cell the opportunity to regulate outer membrane lysis."
"A mechanism has been proposed that would protect mitochondria against outer membrane lysis and inner-membrane domain mixing during crista swelling: fusion of tubular cristae to form larger cristae more adaptable to volume changes. Crista fusion was suggested by the first EM tomograms of mammalian mitochondria, which revealed complex cristae with tubular and lamellar regions (Mannella et al., 1997; Perkins et al., 1997). Larger cristae are more prevalent in condensed mitochondria; decreased matrix volume brings cristae into closer proximity, favoring fusion (Mannella et al., 2001). It is likely that crista fusion in response to matrix contraction is quite extensive. Condensed liver mitochondria have large dilated cristae with multiple (up to seven) junctions (Mannella et al., 1997) and condensed yeast mitochondria may have a single dilated internal cavity with much of the inner membrane pulled away from the outer membrane and no well-defined crista junctions or cristae (Mannella et al., 2001)."
-
Hydrogen economy in glycolysis (the charges may be ignored):
Out (–) In (+) HK H⁺ PFK H⁺ 2GAPDH *2H⁻ 2H⁺ 2ENO 2H₂O 2PK 2H⁺ Total 10H 2H (*2H⁻ from 2NADH)
The expectation (–10H + 2H = –8H) is different from the actual change (–6H), when we obtain the pyruvates:
Toxin Formula Glucose C6H12O6 (–8H →) Expected C6H4O6 (–6H →) 2Pyruvate C6H6O6 2Lactate C6H10O6 2Lactic acid C6H12O6 What explains the difference (between expected and actual change) is that the 2H⁺ released at the dehydrogenase level (GAPDH) are derived from inorganic phosphate rather than extracted from the glucose metabolite (
NADH + H⁺). When we disconsider these 2H⁺, we arrive on the –6H needed.
As for lactate, when it's formed, 4H are consumed from 2H⁻ + 2H⁺. Going backwards, it would be equivalent to never having produced:
- H⁺ (HK)
- H⁺ (PFK)
- 2H⁻ (2GAPDH)
Then, we're left with –2H for 2 lactates (C6H10O6) to match with a glucose molecule (C6H12O6).
Even though 2H⁺ were disconsidered previously, they still occur, just not extracted from glucose metabolites. After canceling out the 3 products listed above, we get:
Out (–) In (+) 2GAPDH 2H⁺ 2ENO 2H₂O 2PK 2H⁺ Total 6H 2H When lactate is exported from the cell, it tends to bring a H⁺ along as counter-ion. So, 2 lactates would take out the equivalent to those 2H⁺ produced at 2GAPDH.
What remains is the bottom of the original table:
- 2H₂O out (produced)
- 2H⁺ in (consumed)
Therefore, lactate synthesis and export is an alkalinizing process for the cell:
- H⁺ are consumed when pyruvates are formed (pyruvate enol-phosphate + H⁺ → pyruvate), to then become lactates to be exported. In contrast, the H₂O molecules produced are neutral.
- LDH reaction consumes H⁺ directly (pyruvate + NADH + H⁺ → lactate + NAD⁺)
- Additional H⁺ tend to be eliminated with lactates as counter-ions (lactate + H⁺)
Nevertheless, lactate is associated with acidification for a few reasons that I'm aware of:
The coexport with a H⁺ alkalinizes the interior and acidifies the exterior of the cell. It's easier to monitor the outside, so that's what we associate with.
Once this lactic acid equivalent leaves the cell, the H⁺ is quickly buffered, leaving lactate to pair with available cations, such as Na⁺ ('HLac' + NaHCO3 → NaLac + H2CO3). If not by using up hydrocarbonate ions, a surge of lactate with expansion of the pool of organic anions can lead to an increase in cations to maintain ion neutrality. This tends to reflect on the concentration of free H⁺, that may rise in proportion along.
Marked ATP hydrolysis, that often coincides with excess lactate formation, is acidifying.
- ATP + H₂O → ADP + Pi + H⁺
Rather than the theoretical source from the previous section, this H⁺ can be the pair for lactate.
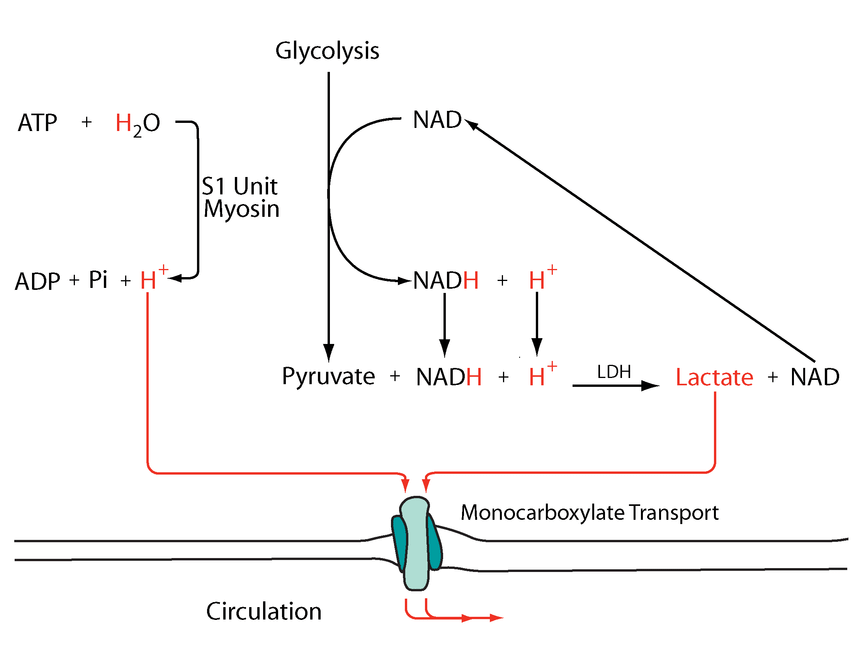
The following diagram has these events simplified:
Metabolic acidosis and fatigue: Where to from here?
-
Observe the inconsistency in the conventional nomenclature for glycolysis metabolites:
Abbreviation Metabolite GLC Glucose G6P Glucose-6-phosphate F6P Fructose-6-phosphate F1,6BP Fructose-1,6-bisphosphate DHAP Dihydroxyacetone-3-phosphate G3P Glyceraldehyde-3-phosphate 1,3BPG 1,3-Bisphosphoglycerate 3PG 3-Phosphoglycerate 2PG 2-Phosphoglycerate PEP Phosphoenolpyruvate PYR Pyruvate I think that they put phosphate in evidence when the molecule becomes a phosphate donor, similar to creatine (phosphocreatine, which is creatine phosphate), but to relegate the core is confusing, and it's worse without bolding.
Standardizing against the norm makes it easier to grasp:
Abbreviation Metabolite GLC Glucose G6P Glucose 6-phosphate F6P Fructose 6-phosphate F1,6BP Fructose 1,6-bisphosphate DHAP Dihydroxyacetone 3-phosphate G3P Glyceral 3-phosphate 1,3BPG Glyceral 1,3-bisphosphate 3PG Glycerate 3-phosphate 2PG Glycerate 2-phosphate PEP Pyruvate Enol-phosphate PYR Pyruvate The numbers next to phosphate indicate the carbon position where phosphates are attached to the molecule.
- For a molecule with 6 carbons, we can infer that a '6-phosphate' is a a phosphate attached to one extremity.
- When we get '1,6-bisphosphate', the molecule contains phosphates on both extremities.
In working with split molecules of 3 carbons, the same approach applies.
- Bis- and tris-: separate (example: P-Molecule-P)
- Di- and tri-: in sequence (example: Molecule-PP)
High-energy phosphate | Wikipedia
"Often, high-energy phosphate bonds are denoted by the character '~'. In this "squiggle" notation, ATP becomes A-P~P~P. The squiggle notation was invented by Fritz Albert Lipmann, who first proposed ATP as the main energy transfer molecule of the cell, in 1941. Lipmann's notation emphasizes the special nature of these bonds. Stryer states:
ATP is often called a high energy compound and its phosphoanhydride bonds are referred to as high-energy bonds. There is nothing special about the bonds themselves. They are high-energy bonds in the sense that free energy is released when they are hydrolyzed, for the reasons given above. Lipmann's term "high-energy bond" and his symbol ~P (squiggle P) for a compound having a high phosphate group transfer potential are vivid, concise, and useful notations. In fact Lipmann's squiggle did much to stimulate interest in bioenergetics."
Number of carbons Saccharide (-ose*) 6 Hexose 5 Pentose 4 Tetrose 3 Triose 2Diose1Monose*As in Alberto's 'godnose'.
The upper part of the glycolysis list are the hexoses (until F1,6BP). After splitting (below F1,6BP), we get the trioses. A hexose or triose phosphate is self-explanatory.
When glucose (6-phosphate) is diverted from glycolysis for nucleotide synthesis, it undergoes oxidative decrapoxylation (losing a carbon), becoming a pentose. It gives name to the Pentose Phosphate Pathway (sometimes referred to as Hexose Monophosphate Shunt).
-
ATP use:
- ATP + H₂O ⇉ ADP + Pi + H⁺
And 2 methods of regeneration:
'Substrate-level' phosphorylation:
- ADP + R-OP + *H⁺ → ATP + R-OH
(R for "Root")
Oxidative phosphorylation:
- ADP + Pi + H⁺ → ATP + H₂O
*Whether a H⁺ is consumed will depend on the reaction.
Creatine cycling does consume (→) and produce (←) H⁺:
- ADP + (Creatine-OP) + H⁺ ⇄ ATP + (Creatine-OH)
Throughout cellular respiration, it's tricky..
Enzyme Participants Dir. Participants HK ADP + R-OP + H⁺ ← ATP + R-OH PFK ADP + R-OP + H⁺ ← ATP + R-OH PGK ADP + R-OP + H⁺→ ATP + R-O⁻ PK ADP + R-OP + *H⁺→ ATP + R-O *But a hydrogen is incorporated elsewhere (R-CH₂ → R-CH₃):
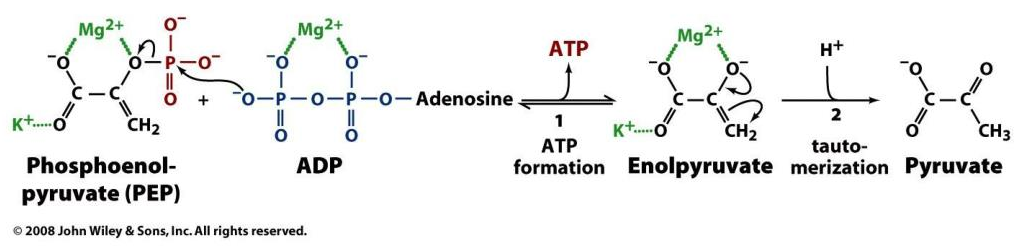
We could argue that glycolysis metabolites become ionized as soon as phosphate is attached to them (early on in glycoylsis), and this contributes to their trapping in cells. However, the definite ionization occurs when phosphates start to be detached in PGK, where crapoxylates are formed (note in the table how H⁺ aren't consumed against expectation).
It's for the phosphorylation steps that you'll find a one-way arrow in diagrams, deeming them irreversible (except for PGK). However, if this was the case in all tissues, glucose resynthesis wouldn't be possible. As an example, the reactions of HK and PFK can be undone through phosphorylases that work as hydrolases:
- Glycolite-OP + H₂O → Glycolite-OH + Pi
⠀
In oxidative phosphorylation, the PO₃⁺ (of inorganic phosphate; Pi) goes towards ADP, and OH⁻ (also from Pi) combines with H⁺ to form H₂O.
- ADP + Pi + H⁺ → ATP + H₂O
With substrate-level phosphorylation in mitochondria, we have a variation of it. SCS reaction (omitting the succinyl group):
- 'ADP' + Pi + CoAS⁻ → 'ATP' + CoASH
As before, the PO₃⁺ (of inorganic phosphate; Pi) goes towards ADP, but here OH⁻ doesn't combine with H⁺ to form H₂O. Rather, oxygen gets incorporated to yield succinate, and H⁺ is accepted by CoA instead of released immediately in free form. No additional H⁺ is consumed.
⠀
Therefore, lactate formation aside, increase reliance on substrate-level phosphorylation for ATP resynthesis goes without the immediate compensatory H⁺ consumption, although complete metabolism should be an overall H⁺-consuming process.It's worth noting the complicators.
Free phosphates will occur as mixed species that take up more or less H⁺ depending on its concentration, and differences in acidity between compartments will affect the composition.
We also know that most of the ATP is produced in mitochondria and consumed in the cytosol. The synthesis of ATP with H⁺ consumption occurs in a more alkaline environment relative to where most of ATP is hydrolyzed. At some stage, the extra H⁺ taken up by phosphates in the cytosol will have to be released when the phosphates in question return to mitochondria. -
@Amazoniac Thanks!
It is on my reading list now! Main goal not to side-track
-
Does Aerobic Respiration Produce Carbon Dioxide or Hydrogen Ion and Bicarbonate?
The majority of CO₂ in the body circulates as the hydrocarbonate ion:
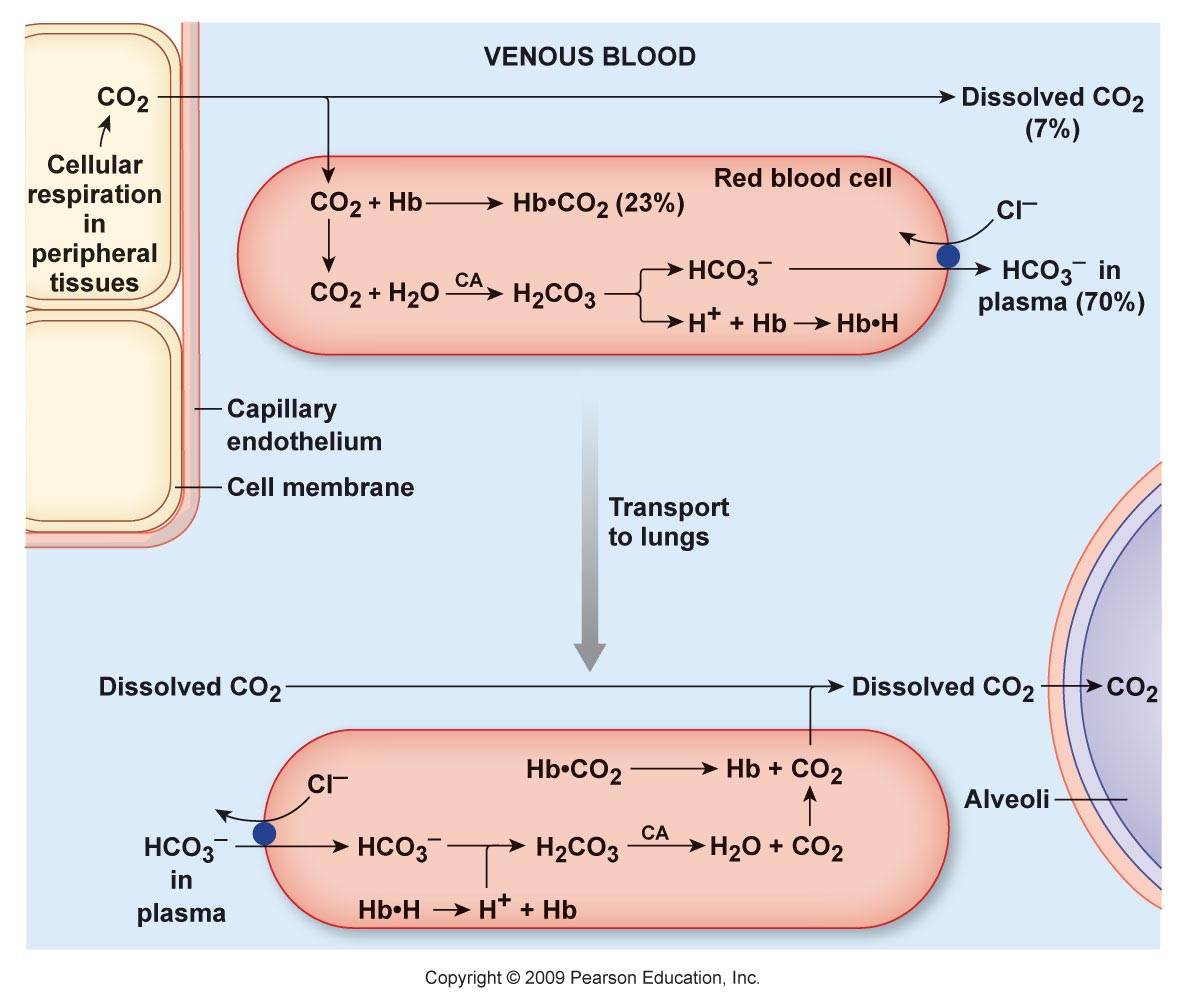
Carbon dioxide and derivatives Content Hydrocarbonate ion (HCO₃⁻) ~70% Carbamates (protein-bound; R-NH-CO₂) ~23% Carbon dioxide (CO₂) ~7% Carbonic acid (H₂CO₃) <1% Organic anions are considered alkalinizing when they are hydrocarbonate precursors. However, prior to its formation, CO₂ has to be hydrated, and for every hydrocarbonate ion derived from CO₂, a H⁺ is released in the system:
- CO₂ + H₂O ⇄ H₂CO₃ ⇄ H⁺ + HCO₃⁻
The H⁺ are temporarily sequestered, but complexation doesn't negate it.
It's easy to overlook the H⁺ load for not being apparent in circulation, where normal levels are:
- [HCO₃⁻]: 22-32 mmol/L
- [H⁺]: 0.000036-0.000043 mmol/L (from pH: 7.44-7.37)
Far from a 1:1 ratio.
It can be argued that hydrocarbonate precursors alkalinize for consuming H⁺ in priming molecules for complete oxidation (into CO₂ and H₂O), which is true, but the consumption is compensated when the equivalent of carbonic acid molecules appear in the system.
Unlike non-volatile acids that can remain paired by a corresponding counter-ion until elimination (example: sodium sulfate), the occurrence of carbonic acid or variants yields CO₂ and H₂O. This CO₂ leaves the body, and the original pairing cation is left as unpaired as residue (example: sodium
citrate).To maintain ion neutrality and rebalance, the body may try to lower cations, which would affect H⁺ concentration, and conserve anions, including hydrocarbonate. Fluid redistribution from the excess cation can also dilute H⁺. The hydrocarbonate are first and foremost carbonic acid precursors, so we need explanations along these lines.
@Lejeboca said in On Cellular Organization and Respiration:
@Amazoniac Thanks!
It is on my reading list now! Main goal not to side-track
Спасибо за визит.
-
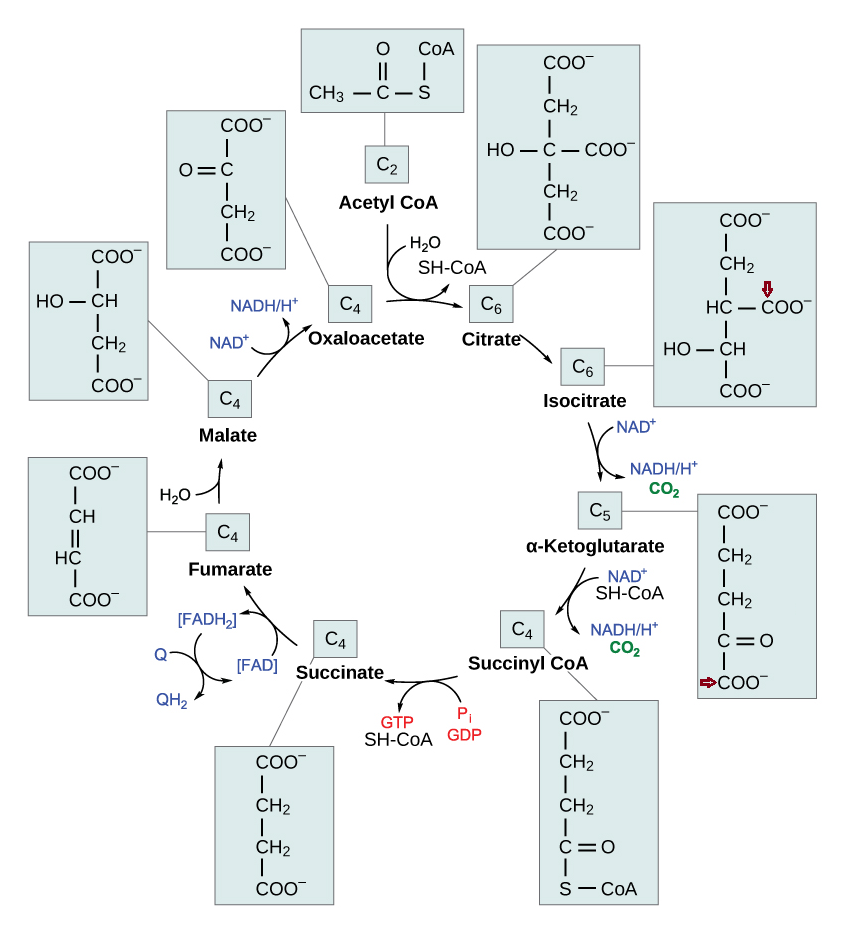
Each turn of the TCA cycle eliminates 2 carbons in consecutive (oxidative) decrapoxylation steps:
- IDH: isocitrate → ketoglutarate* + CO₂
- KGDH: ketoglutarate* → succinyl(CoA) + CO₂
A peculiarity of the TCA cycle is that succinate and (its product) fumarate are symmetrical molecules (⇈). Since this property applies to both of them, we can tell that succinate dehydrogenase modifies succinate evenly.
The next reaction is the conversion of fumarate to malate, where asymmetry appears. Even though fumarate is symmetrical, it contains newer or older atoms throughout the molecule. Depending on which side of fumarate is primarily changed after fumarase, the carbon stay in the cycle will differ.
This gives an idea:
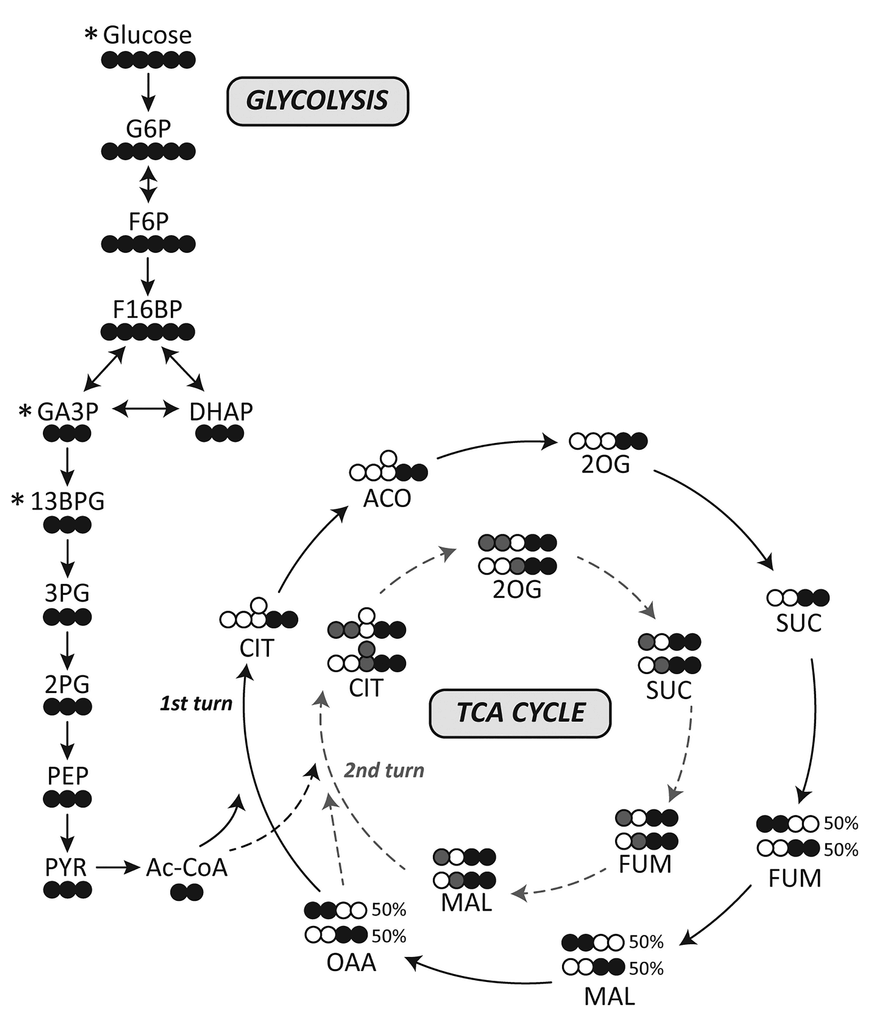
I've adapted it for ease of tracing and until completion:
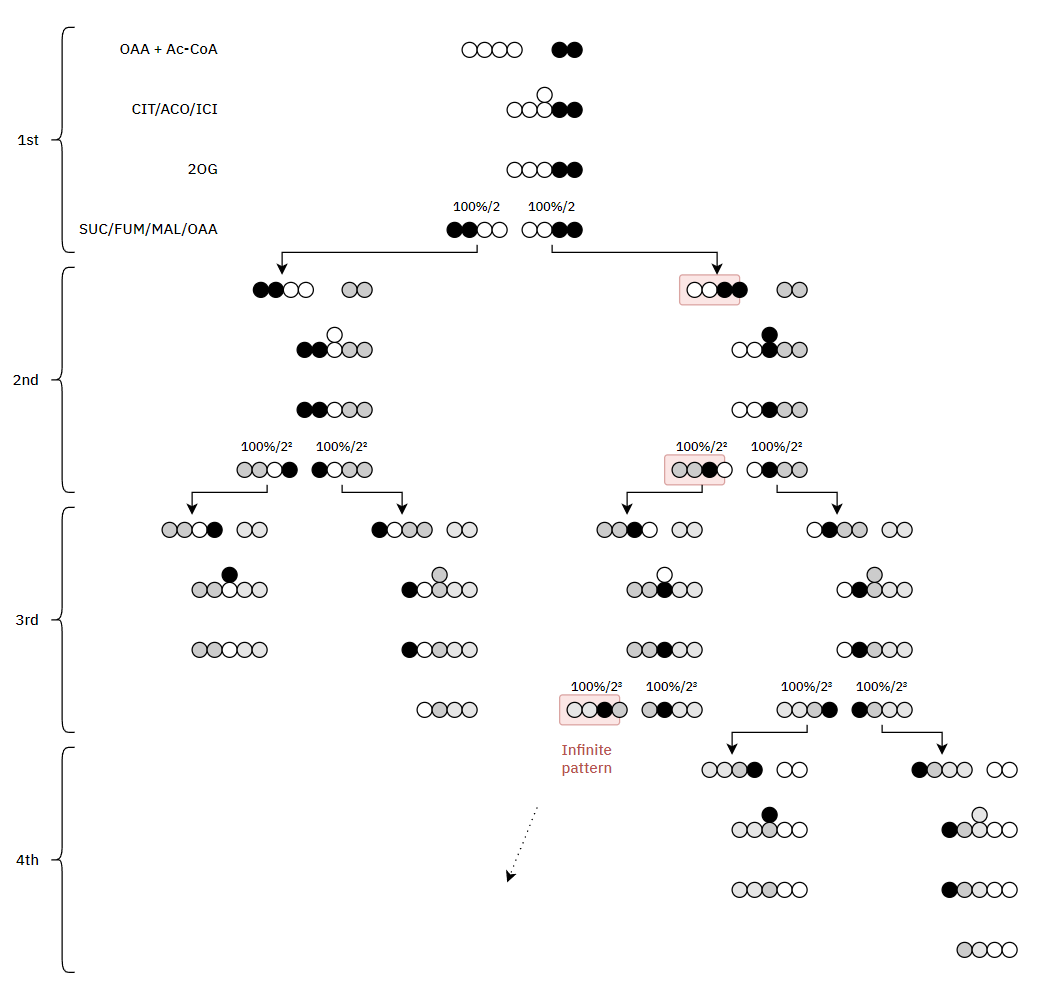
Therefore, the original carbons (from a given acetyl group) aren't eliminated straight away. They remain intact on the 1st turn, and the chances of their complete elimination appear to be:
- 50% on the 3rd turn
- 25% on the 4th turn
- 12.5% on the 5th turn
- 6.25% on the 6th turn
- ...
*Ketoglutarates:
- 2-oxoglutarate = a-ketoglutarate
- 3-oxoglutarate = b-ketoglutarate
Alpha refers to the second carbon because the first is the crapoxyl group, that's disconsidered in counting (similar to beta-oxidation).
Urine Organic Acids as Potential Biomarkers for Autism-Spectrum Disorder in Chinese Children
"[..]3-oxoglutarate, a common metabolite of yeast and fungi (Thomas et al., 2010; MacFabe et al., 2011; Kocovska et al., 2012), was significantly lower in children with autism. The low concentrations of both carboxycitric acid and 3-oxoglutarate that we observed in urine from autistic patients could be due to increased uptake of these compounds across the blood-brain barrier of the brain. Our results are consistent with previous studies that showed anti-fungal treatments for children with autism can effectively reduce the amounts of corresponding organic acid indicators (Cobb and Cobb, 2010), and suggests that gastrointestinal yeast could provide a basis for dietary adjustments such as gluten/casein-free diets that are important for children’s nervous system development and could mitigate autism symptoms. 3-oxoglutarate in urine is associated with the presence of harmful gut flora such as Candida albicans (Schmidt, 1994)."
It's fine to omit the 'alpha' prefix from ketoglutarate for the same reason that we only need to clarify which Paris we're going to when it's not the one in France.
-
At last, an objective comparison of dietary toxins:
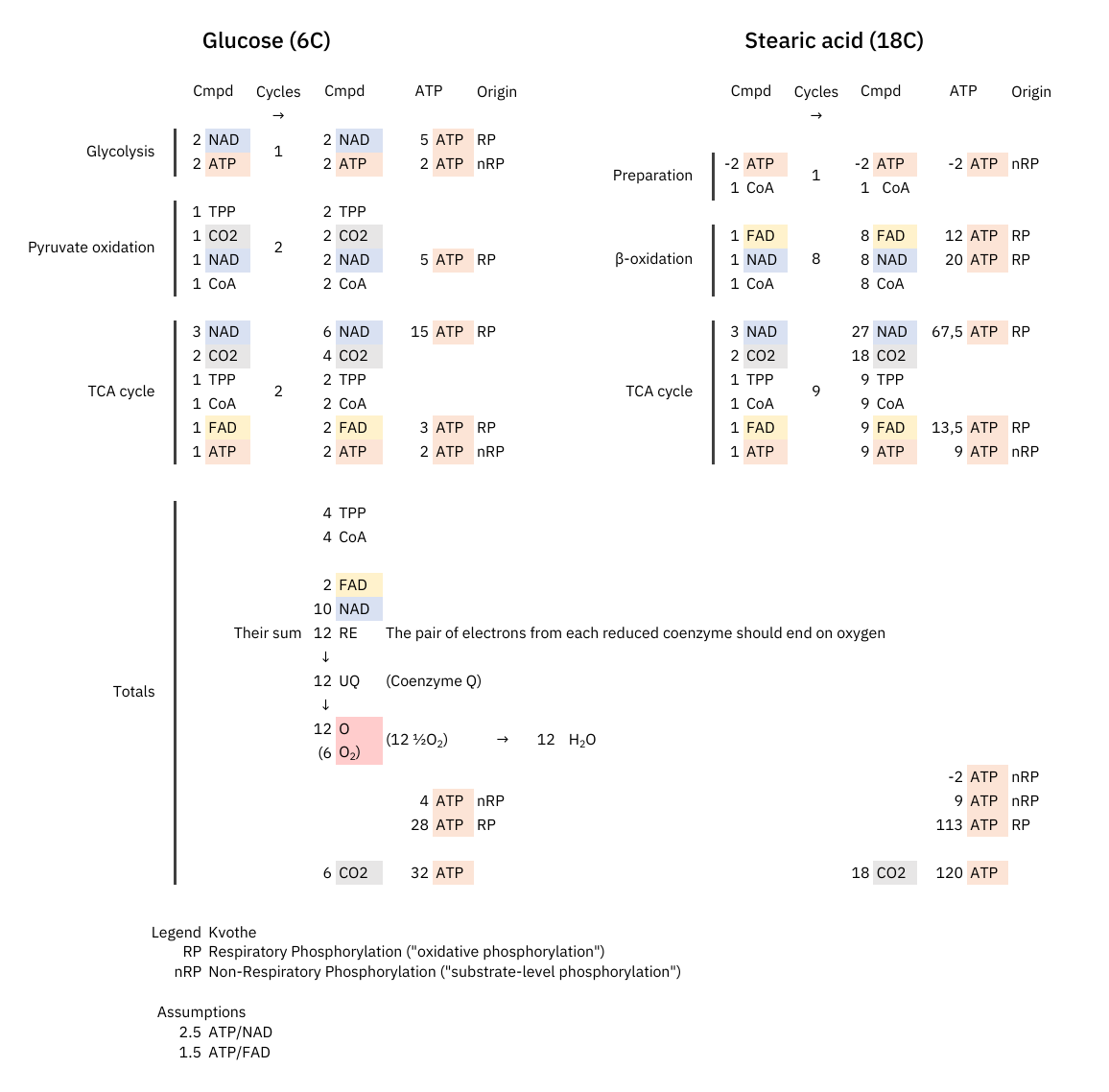
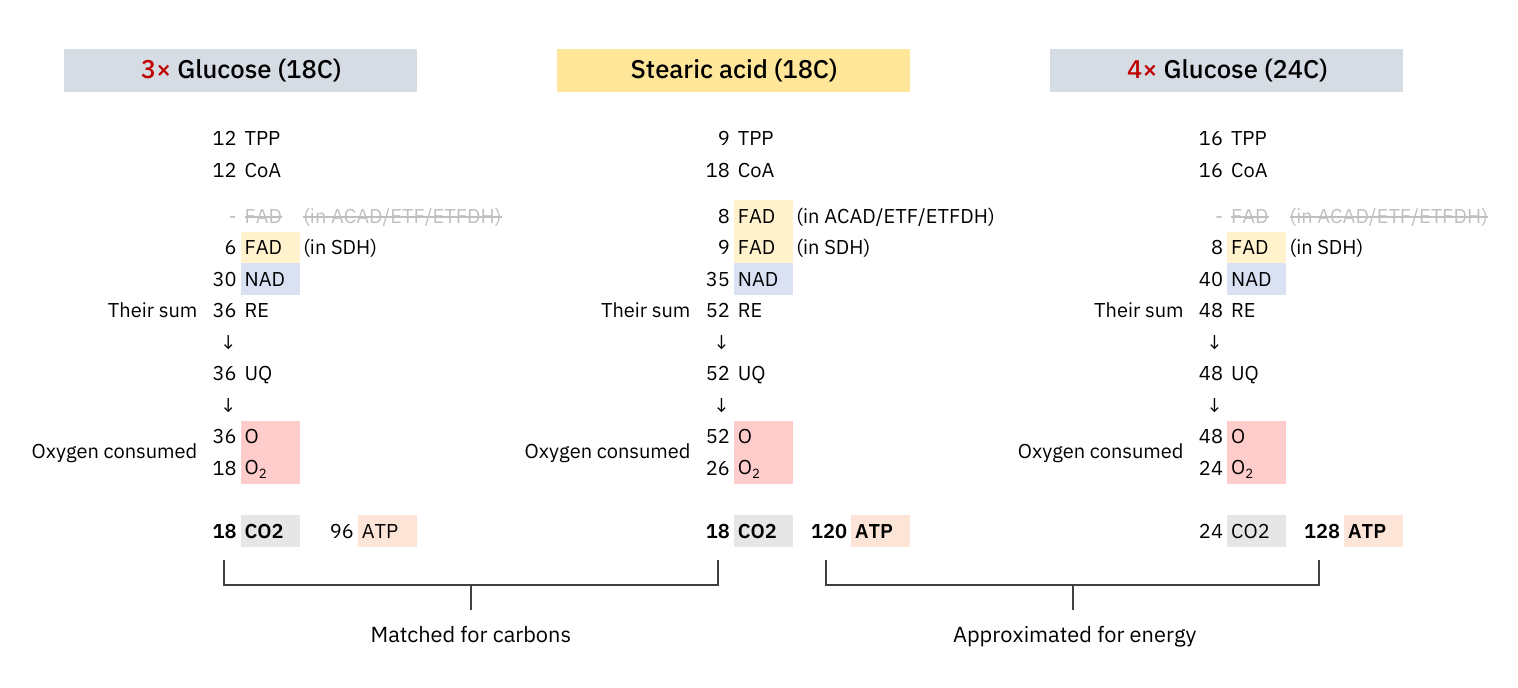
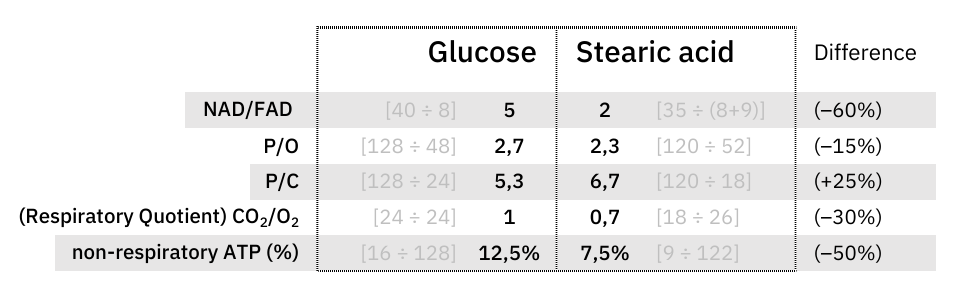
Since these coenzymes are recycled, the most expensive phase with them must be in adapting to different needs. Once the cell becomes adapted, it's a matter of replenishing obligatory losses. I've omitted some coenzymes for clarity.
The comparison makes it clear once again that the oxidation of glucose doesn't produce more CO₂ per molecule metabolized than fatty acids.
What explains the CO₂ effect is a difference in ATP production: fatty acid oxidation can yield about 25% more ATP than glucose on a carbon basis (P/C), resulting in less exposure to CO₂ to produce the same energy.
The NAD/FAD ratios in their complete catabolism:
- NAD 5:1 FAD -- Glucose
- NAD 2:1 FAD -- Fatty acids (rounded for common ones)
Someone may associate FAD with Complex II and have with the impression that fatty acid oxidation overwhelms Complex II. However, if we match substrates for energy yield and argue that glucose oxidation exposes the person to more CO₂, we must be consistent with the matching in other comparisons.
We're dealing with different pools of FAD:
-
FAD within the ACADH-ETF-ETFDH complex (more about it below) is responsible for the marked decrease in the NAD/FAD ratio;
-
FAD within SDH (Complex II) remains similarly affected by glucose and fatty acids.
These are separate routes of transferring electrons.
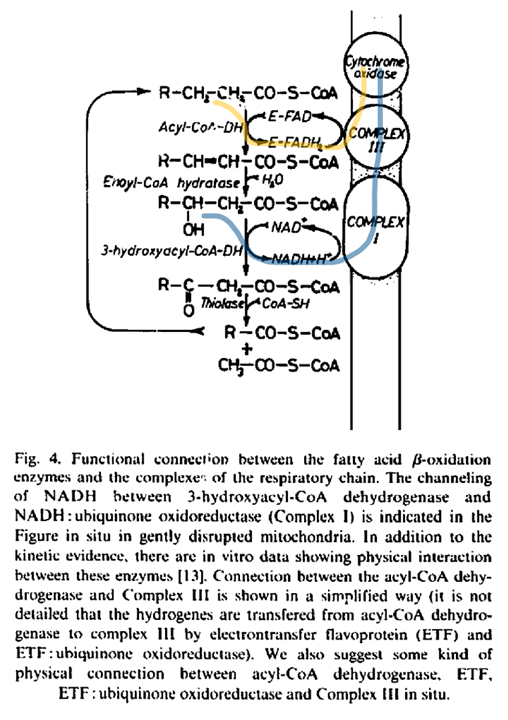
With a focus on the first mentioned route, the middle row shows the 4 enzymes of a standard β-oxidation cycle:
Lack of Myoglobin Causes a Switch in Cardiac Substrate Selection
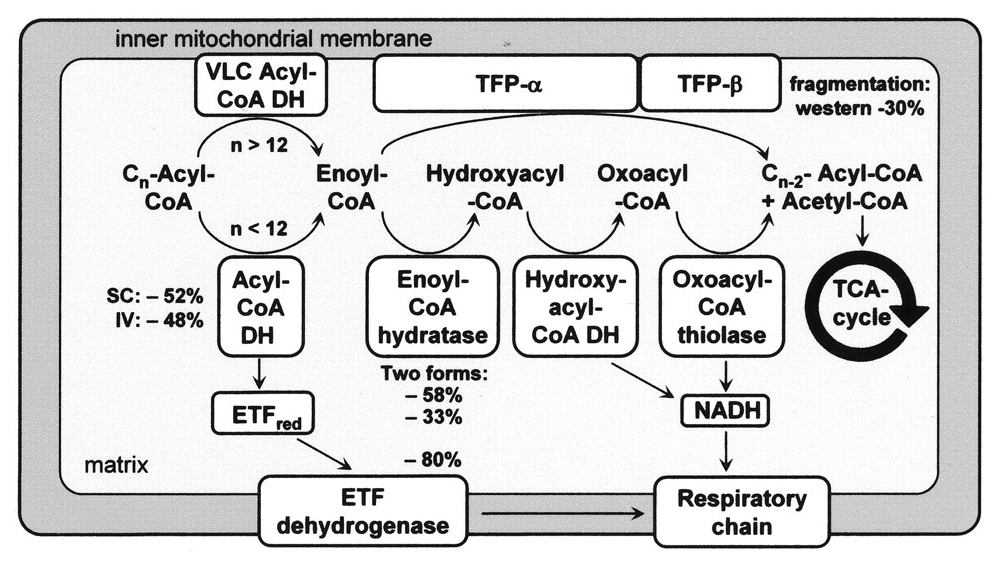
Abbreviation Enzyme Function ACADH Acyl-CoA dehydrogenase Oxidation ..and the Trifunctional Protein (TFP) set: ECH Enoyl-CoA hydratase Hydration HADH Hydroxyacyl-CoA dehydrogenase Oxidation KAT Ketoacyl-CoA thiolase Thiolysation The bottom row of the image shows the respiratory complexes.
In between the middle and bottom rows, linking β-oxidation to respiratory dehydrogenases, are the mobile ETF and NAD molecules.
- ACADH → ETF → ETFDH
- HADH → NAD → Complex I
ETF stands for Electron-transfer Flavoprotein, which is carrier molecule and the FAD equivalent in purpose to NAD.
ETFDH (ETF Dehydrogenase) is a respiratory complex that metabolizes ETF. It's sometimes called ETF:Q-O (ETF-to-Quinone Oxidoreductase).
FAD occurs embedded in ACADH, and the same applies to each of these interacting proteins:
- ACADH (FAD) → ETF (FAD) → ETFDH (FAD)
It's fad everywhere, resembling a thiamin overdose support group.
The paths of these β-oxidation dehydrogenases (ACADH and HADH) eventually converge in ubiquinone.
FAD-dependent NAD-dependent β-ox. Enzyme ACADH HADH Carrier ETF NAD Resp. Enzyme ETFDH (ETF:Q-O) Complex I (NADH:Q-O) Carrier ⤷ UQ ⤶ Resp. Enzyme Complex III Carrier Cyt c Resp. Enzyme Complex IV Therefore, the extra FAD cycled in fatty acid oxidation doesn't involve SDH (Complex II), but the ACADH-ETF-ETFDH complex featured above.
Since every acetyl-CoA that's metabolized in the TCA cycle is derived oxidatively from a β-oxidation cycle, we'll have a succinate (from TCA cycle → SDH) molecule for every ETF (from β-oxidation → ETFDH); so, the different routes distribute electrons somewhat evenly between them, without burdening Complex II.
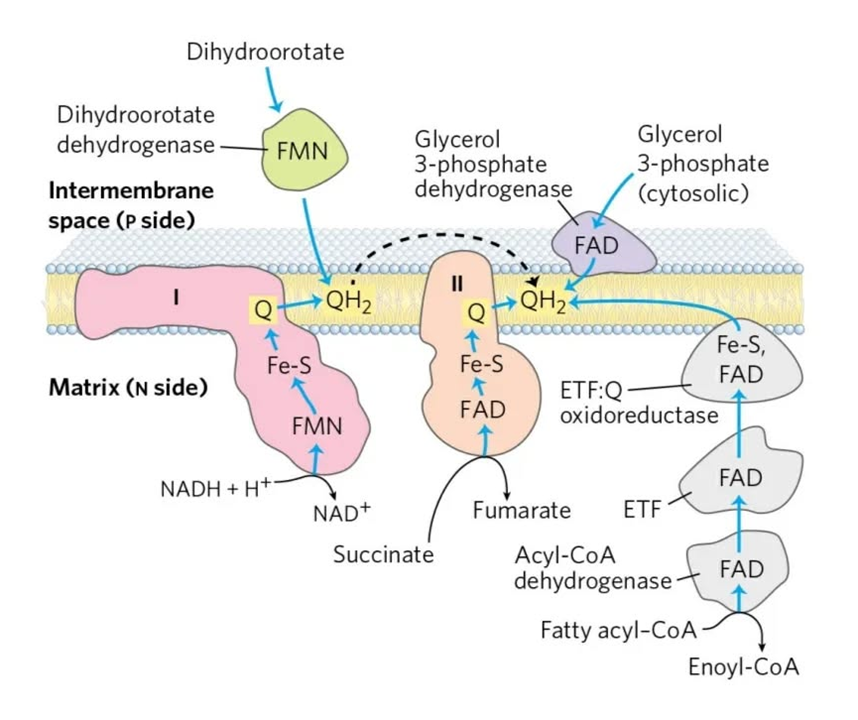
⠀Source: the internet, but possibly Albie's Principles of Biochemistry.
It's an almost even distribution because the last acetyl-CoA isn't derived oxidatively, but as a leftover product of the final β-oxidation cycle, which explains one less FAD needed in ACAD/ETF/ETFDH (check the original tables).
As a side note, the catabolism of certain amino acids and choline is also dependent on ETF-ETFDH.
Electron transfer flavoprotein and its role in mitochondrial energy metabolism in health and disease
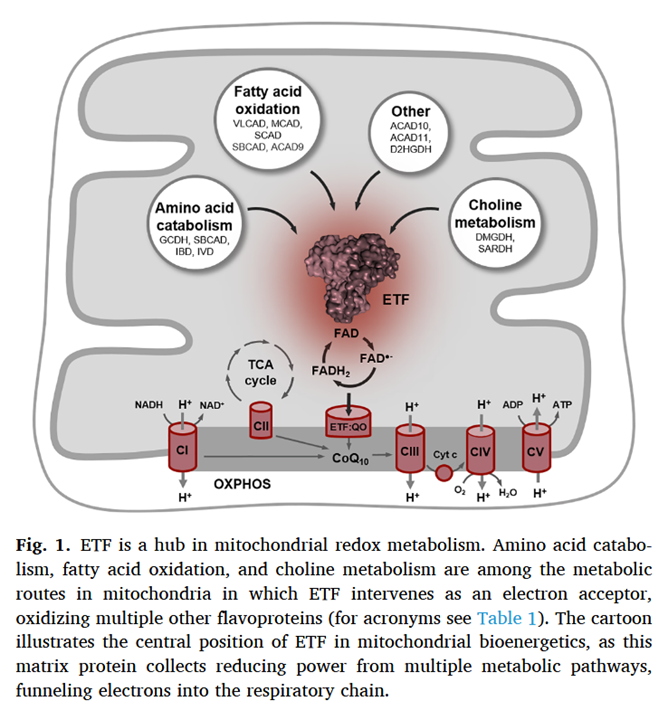
Along the lines of taking energy into account, the ratio of ATP produced to oxygen consumed (P/O) indicates how fatty acid reliance needs less oxidation than anticipated, it's not too far behind glucose.
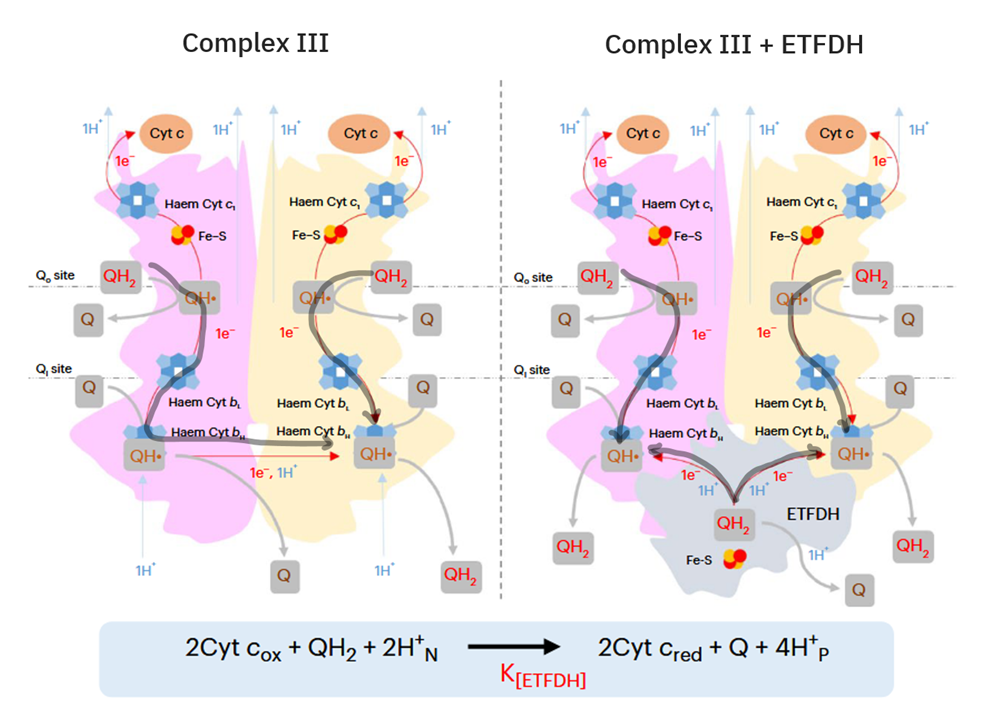
In addition, the components related to β-oxidation can cluster with the respiratory complexes to maximize efficiency and minimize damage. 'Respirasome' (CI + CIII₂ + CIV) is a known respiratory supercomplex, but we also have lesser-known associations, one of them coordinating ETFDH with CIII, while ACADH and ETF likely remain nearby.
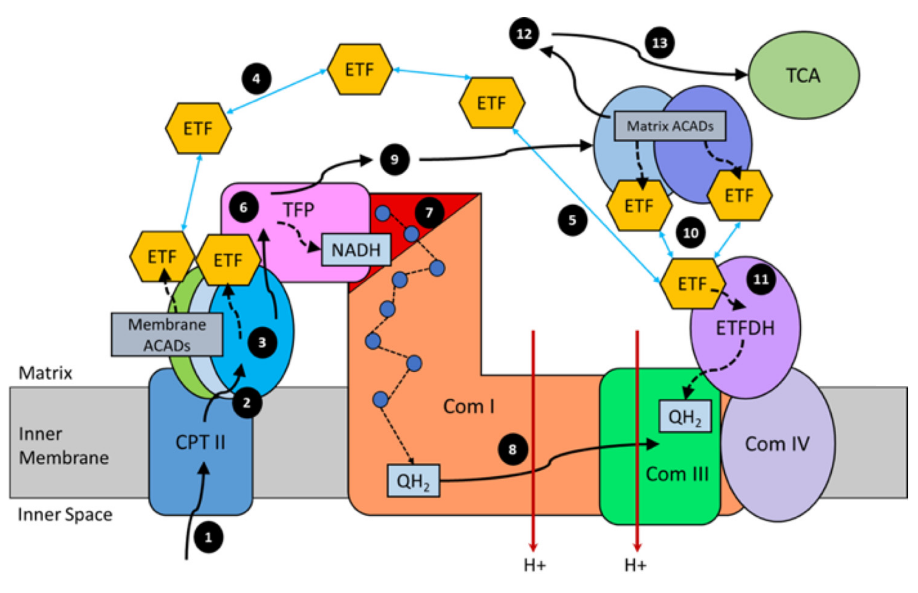
"For steps 1–3, long-chain acyl-CoA substrates are transferred into mitochondria as acylcarnitines, which cross from the intermembrane space into VLCAD through CPTII in the inner membrane. VLCAD then accepts and catalyzes the released long-chain acyl-CoA substrate to its enoyl–CoA product with reduction of ETF. The protein complex promotes metabolite channeling for all these reactions.
For steps 4 and 5, reduced ETF is released from VLCAD into the mitochondrial matrix, where it is free to find its redox partner, ETFDH, and shuttle its reducing equivalents (QH2) to ETC complex III. Alternatively, for high catalytic efficiency of transfer of electrons from FAO to ETC, the ETF may remain associated with the macromolecular FAO–ETC complex and instead slide down the membrane-associated proteins to more efficiently contact ETFDH.
For steps 6 and 7, long-chain enoyl–CoAs, from VLCAD, channel directly to TFP where the next three reactions in the cycle occur, producing one molecule of acetyl-CoA and an acyl-CoA substrate that is two carbons shorter. As the acyl-CoAs become shorter, they become more hydrophilic, allowing them to be released into the matrix. NADH generated by the LCHAD reaction of TFP can directly channel to the NADH-binding domain of complex I.
For step 8, in complex I NADH is oxidized through iron–sulfur clusters to generate QH2 at the Q-binding domain, which is then transported through the membrane electron channel of complex I to complex III.
For steps 9 and 10, medium- and short-chain acyl-CoA substrates produced by TFP are transferred to MCAD and SCAD in the matrix or in a more weakly-associating peripheral domain of the multifunctional FAO–ETC complex. ETF is once again reduced and released to ETFDH, as in step 5. The remaining FAO reactions are catalyzed by monofunctional enzymes that are likely also weakly associated at the periphery of the complex.
For step 11, ETFDH oxidizes reduced ETF by reducing CoQ to QH2, which is then channeled to ETC complex III.
Finally, the acetyl-CoA generated by FAO is free to enter the TCA cycle or to be utilized for ketone body production (steps 12 and 13)."
For an overview:
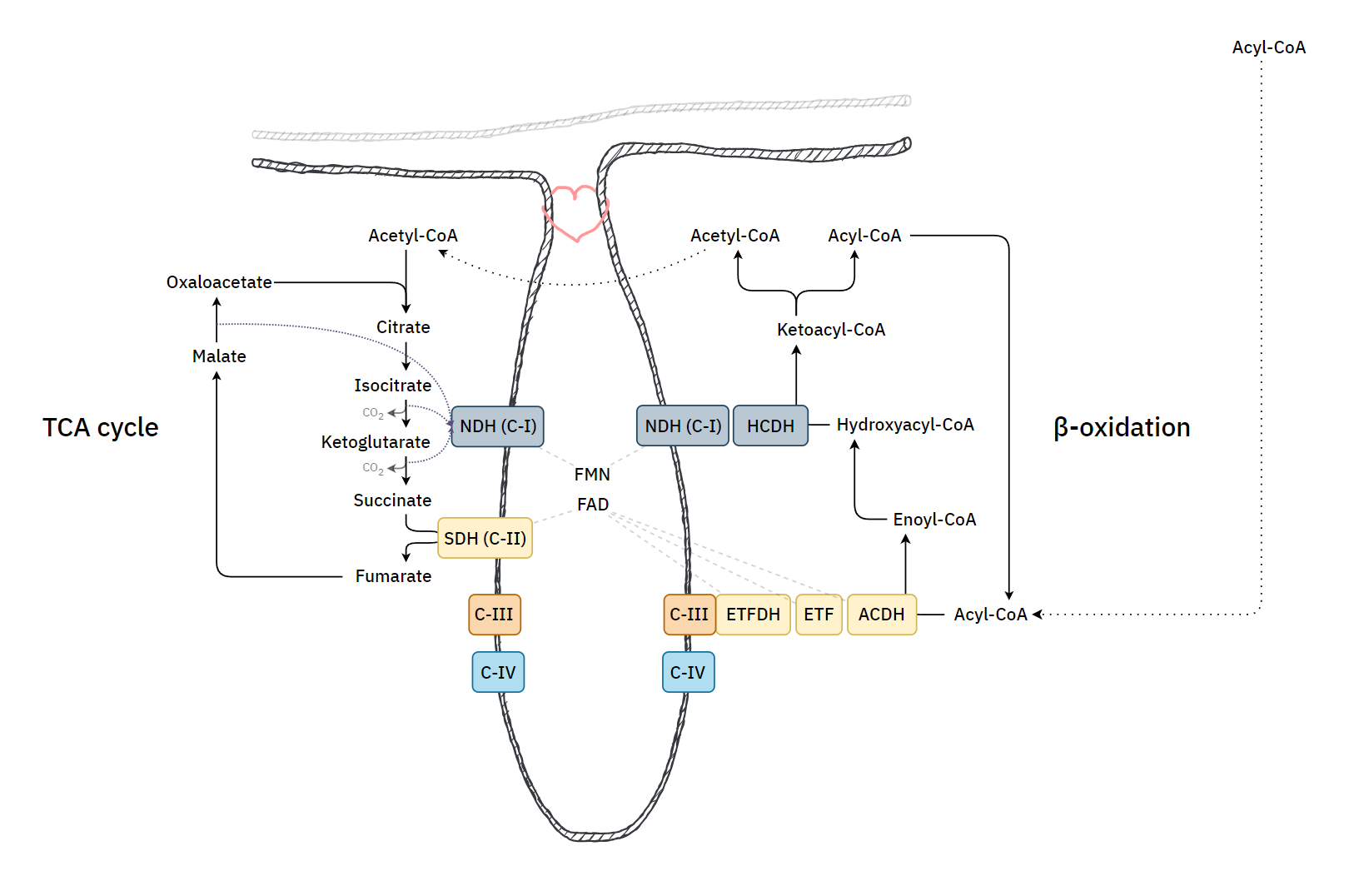
If the cell starts to rely more on fatty acid oxidation and there is an excess of ubiquinol relative to Complex I use, electrons can flow back, leading to the formation of 'reactive oxygen species' and a purposeful disintegration of Complex I. This frees the associated Complex III to reorganize supercomplexes in a way that meets a different demand. Therefore, where fatty acid oxidation is dangerous, the body tries to regulate it as drugs.
-
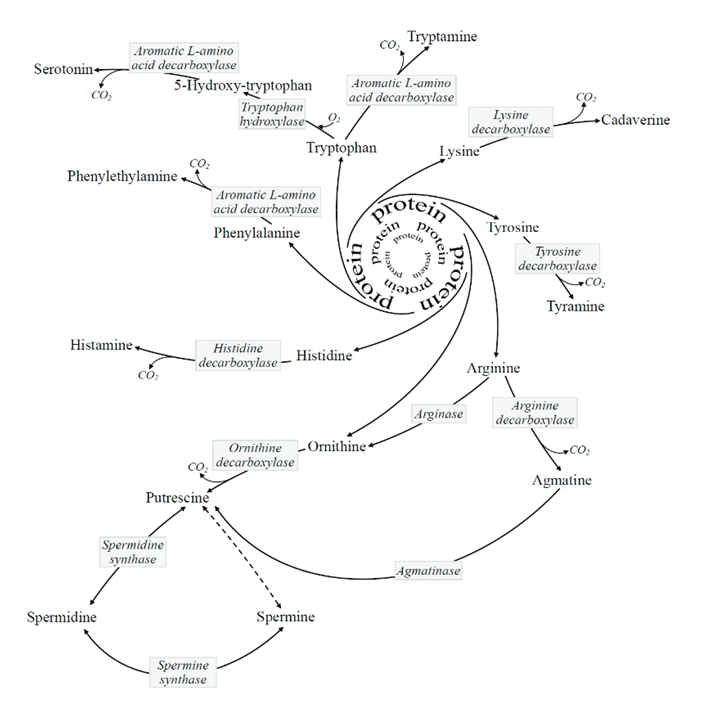
And an incomplete list of unexpected CO₂ sources:
Process Enzyme Reaction Pentose Phosphate Pathway PGDH Hexose (6C) → pentose (5C) + CO₂ (1C) Fatty acid synthesis KAS* Malonyl (3C) + acetyl (2C) → ketobutyryl (4C) + CO₂ (1C) Acetone synthesis - Acetoacetate (4C) → acetone (3C) + CO₂ (1C) Oxoglutarate oxygenases OGO Oxoglutarate (5C) → succinate (4C) + CO₂ (1C) Pyruvate synthesis from malate ME Malate (4C) → pyruvate (3C) + CO₂ (1C) PEP synthesis PEPCK Oxaloacetate (4C) → pyruvate-enol-phosphate (3C) + CO₂ (1C) Glycine cleavage system GLDC Glycine (2C) + THF (+0C) →→ methylene-THF (+1C) + CO₂ (1C) Coenzime A synthesis PPCD PPC (12C) → P4P (11C) + CO₂ (1C) Aldehyde dehydrogenase ALDH formyl-THF (+1C) → THF (+0C) + CO₂ (1C) Taurine synthesis CSAD CSA/CA (3C) → hTau/Tau (2C) + CO₂ (1C) Cholesterol synthesis PPMD Mevalonate-5-diphosphate (6C) → Isopentenyl diphosphate (5C) + CO₂ (1C) Polyamine synthesis SAMD SAM (–0C) → dcSAM (–1C) + CO₂ (1C) Other biogenic amine syntheses (AA decarboxylations) † *Preceded by carbon incorporation: gain followed by loss. But remains a reaction that releases carbon dioxide.
†Biochemical and Pharmacological Properties of Biogenic Amines
-
Miguel Favo released a video titled:
He runs with core misconceptions that are widespread and worth addressing, appearing to closely adhere to the 'new head, old hat' publication.
"[..]you're basically storing electrons in [NAD and FAD]. Now, [NADH and FADH2] are then brought into the electron transport chain, which is second part of the mitochondria you need to know."
"Now, as I mentioned, NADH goes to Complex I. Well, FADH2 goes to Complex II. When you start to have a lot more electrons flow to FADH2, which you see in fats, because you have only a 2:1 ratio of NADH/FADH2 versus carbs where you have a 5:1 ratio of NADH/FADH2, Complex II starts to hog CoQ10 and steal it from Complex I."
"[..]when you inhibit the fatty acid oxidation and you basically lead to a circumstance where you don't have all the FADH2 going to Complex II and over reducing co-enzyme Q[..]"
The reduced form of FAD in question is not "brought into" or "going to" the Electron-transfer System (ETS), as FAD already belongs to it bound to stationary respiratory complexes.
The intention of highlighting select coenzymes—NAD and FAD—is to differentiate the catabolic yields based on reduction potentials, not to specify the particular electron entry-points, as many respiratory complexes have coenzymes in common. It's important to acknowledge this to avoid limiting FAD to SDH (Complex II), for example.
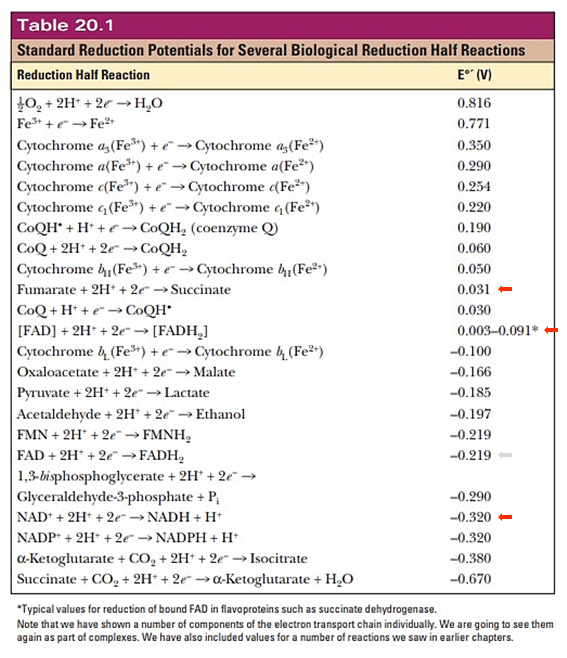
(The reduction potential should vary depending on the flavoprotein, to be compatible with the preceding reaction.)
Each respiratory complex listed below is a flavoprotein:
Mobile e⁻ donor Respiratory complex Contains NAD Complex I FMN Succinate SDH FAD ETF ETFDH FAD Proline PRODH FAD Choline CHODH FAD Sulfide SULDH FAD Glycerol phosphate GPDH FAD Dihydroorotate DHODH FMN Dehydrogenase abbreviations here refer to whole and functional complexes.
For a comparison that specifies the respiratory chain entry-points, yet separated by similarities in reduction potentials:
Dehydrogenases NAD FAD ⠀ Glucose ⠀ Glycolysis GAPDH (→ MAS) Complex I {GADPH (→ GPS)} {GPDH} ⠀ Pyruvate oxidation PDHc Complex I ⠀ TCA cycle IDH Complex I KGDHc Complex I SDH (Cx-II) SDH MDH Complex I ⠀ Total Glucose with MAS 5 1 Glucose with GPS 4221⠀ Fatty acids ⠀ β-oxidation (standard) ACADHs ETFDH HADH Complex I ⠀ TCA cycle IDH Complex I KGDHc Complex I SDH (Cx-II) SDH MDH Complex I ⠀ Total Fatty acid oxidation 4221⠀ Ketones ⠀ Ketolysis HBDH Complex I ⠀ TCA cycle (as above) 3×Complex I SDH ⠀ Total Acetoacetate 3 1 Hydroxybutyrate 4 1 Summary (still with rounded values):
Complex I (NDH) Complex II (SDH) Complex IIS* (ETFDH) Glucose 5 1 - Fatty acid 4 1 1 *Doing a fun with Complex 2.5.
It's their grouping that gives the usual ratios:
- NAD 5:1 FAD -- Glucose
- NAD 2:1 FAD -- Fatty acids
- [4:(1+1)]
Therefore, the main change responsible for the marked decrease in the NAD/FAD ratio is the inclusion of ETFDH associated with β-oxidation, making it unreasonable to neglect ETFDH and misattribute its involvement to SDH. ETFDH and SDH differ in their capacity to produce ROS, but to compare them takes the recognition that we're dealing with different respiratory complexes and electron routes. Their particularities invalidate any attempt to suggest that it's fine to treat them as one, without the need to differentiate.
Moreover, observe in the video that the simplified NAD/FAD ratio with glucose ended up right (5:1) only because the missing PDH was compensated for by GAPDH quantified erroneously twice.
Electrons that are pulled off in glycolysis (via GAPDH) commonly reach mitochondria through the malate-aspartate shuttle (MAS), where each imported pyruvate can bring a malate molecule along. Those electrons are then recovered from malate in a NAD-dependent reaction, which is why they're expected to contribute to the NAD-linked respiration.
However, in situations where electrons reach mitochondria through the alternative glycerol-phosphate shuttle (GPS), they enter the respiratory chain on a lower level through a FAD-linked pathway, and the NAD/FAD ratio difference between glucose and fatty acid catabolism disappears.
- NAD 5:1 FAD -- Glucose with MAS
- NAD 2:1 FAD -- Glucose with GPS
- NAD 2:1 FAD -- Fatty acids
Yet, ratios don't mean much on their own. To get to the principle in question, consider these simplified paths:
- NAD → [FMN → FeS]Cx-I → UQ
- Suc → [FAD → FeS]SDH → UQ
- ETF → [FeS → FAD]ETFDH → UQ
If the reduction potential differences happened to be equal, a mere rerouting of electrons (after a partial shift from NAD-linked to FAD-linked respiration) wouldn't be an issue because one route would increase at the expense of the other, without perturbing the ubiquinone pool. Example:
- Situation Aː 65 NAD + 13 FAD → 78 UQ
- Situation Bː ↓52 NAD + ↑26 FAD → 78 UQ
However, it is Complex I, III, and IV that are directly responsible for driving respiratory phosphorylation. FAD-linked substrates have less reducing power, so they enter the 'Electron-transfer System' bypassing Complex I. As a consequence, fewer protons are extruded, leading to a decreased ATP yield per electron or oxygen consumed.
It can be useful to identify respiratory complexes based on their ability to drive proton translocation, either directly (middle column) or indirectly (middle row):
Matrix DHs ↳ NAD 
Complex I PRODH, ETFDH, SDH → ↳ UQ ↴ ← GPDH, DHODH, SULDH, CHODH Complex III ↳ Cyt c ↴ Complex IV (Funky arrows render automatically.)
Now stacking them:
Enzyme Proton (H⁺) translocation Concentrators Complex I 4H⁺/2e⁻ ↴ SDH - ↴ ETFDH - ↴ PRODH - ↴ SULDH - ↴ CHODH - ↴ GPDH - ↴ DHODH - Complex III 4H⁺/2e⁻ Complex IV 2H⁺/2e⁻ Total NAD-linked respiration (w/ Complex I) 10H⁺/NAD FAD-linked respiration (w/o Complex I) 6H⁺/FAD Dissipators Complex V (w/ ATP)* −8H⁺/3ATP PTP-ANT (w/o ATP) −3H⁺/3ATP UCP, NNT, leakage, etc. (w/o ATP) −?H⁺/3ATP Total −11H⁺/3ATP Per each ATP −3.7H⁺/1ATP For each H⁺ −1H⁺/0.27ATP Simplified (−12H⁺/3ATP) −1H⁺/0.25ATP *Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria
Complex V
"Each 360° rotation of the central stalk takes each catalytic site through [..] conformations in which substrates bind, and 3 ATP molecules are made and released."
"The turning of the rotor is impelled by protons, driven across the inner membrane into the mitochondrial matrix by the transmembrane proton-motive force."
"According to current models based on structures, the number of translocated protons for generation of each 360° rotation is the same as the number of c-subunits in the ring, as each c-subunit carries a carboxylate involved in protonation and deprotonation events. In the yeast F-ATPase, the ring has ten c-subunits, and so 10 protons are translocated per 3 ATP molecules made during a 360° cycle; therefore, the bioenergetic cost to the enzyme is 3.3 protons per ATP (9). However, the c-ring sizes differ between species; c10–c15 rings have been found in yeast, eubacterial, and plant chloroplast F-ATPases (10–13)."
"The most important inference from the presence of the c8-ring in bovine F-ATP synthase, is that 8 protons are translocated across the inner mitochondrial membranes per 360° rotation of its rotor. As each 360° rotation produces 3 ATP molecules from the F1-domain, the bioenergetic cost of the enzyme making an ATP is 2.7 protons [from 8H⁺/3ATP]."
Dissipators that don't generate ATP reduce the efficiency of its synthesis, making the process more costly. Relating them in terms of ATP production remains useful, for being the primary purpose of cellular respiration.
Phosphate translocase protein (PTP) can lead to the dissipation of a H⁺ for every Pi imported (with ADP) and ATP synthesized: −1H⁺/1ATP. As shown below:
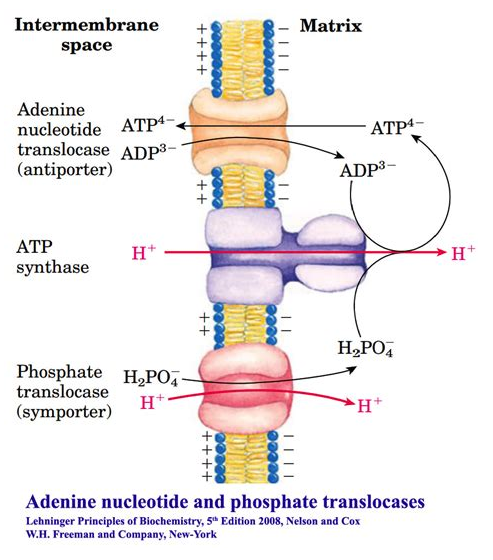
We can also compare their contribution to the cost of respiration. For example:
- 8H⁺ + 3H⁺ = 11H⁺ (100%)
- 3H⁺/11H⁺ = 27% of H⁺ dissipated without ATP synthesis
Given that respiratory complexes prevail in mitochondrial cristae, it's unclear to me how PTP could dissipate H⁺ from an isolated compartment as ADP and Pi return from the cytosol to the matrix of mitochondria. Nevertheless, once these factors are taken into account, it becomes easy to make future adjustments.
From the table:
- 0.25 ATP produced per 1 H⁺ consumed (dissipated): 0.25ATP/H⁺
Then:
- NAD ⇝ 10H⁺
- 10H⁺/NAD × 0.25ATP/H⁺ = 2.5 ATP/NAD
- FAD ⇝ 6H⁺
- 6H⁺/FAD × 0.25ATP/H⁺ = 1.5 ATP/FAD
Because of variation and uncertainties, the values are often given in ranges:
- 2–3 ATP/NAD
- 1–2 ATP/FAD
When cells increase reliance on substrates that depend more on FAD-linked respiration, they have to compensate for the inefficiency by oxidizing more molecules to produce the same amount of ATP. This compensation is what's expected to perturb the ubiquinone pool, where respiratory complexes converge. Reworked example:
- Situation Aː 65 NAD + 13 FAD → 78 UQ
Situation Bː ↓52 NAD + ↑26 FAD → 78 UQ- Situation Cː ↓56 NAD + ↑28 FAD → 84 UQ
But now both situations yielding similar amounts of ATP while demanding different amounts of UQ.
If we focus on FAD changes, we may expect a dramatic perturbation on UQ, but not all of this change will always represent FAD- competing with NAD-linked respiration, as more than half of FAD dependence can increase at the expense of NAD. Decreasing the involvement of Complex I could free up a fraction of UQ for other complexes. From the example:
NAD
- 56 RE − 65 RE = −9 RE
FAD
- 28 RE − 13 RE = +15 RE
Impact on UQ
- −9 RE + 15 RE = +6 UQ
If UQ occurs as a single and shared pool, eventual perturbations would be minimized from this partial Complex I disuse.
Some aspects to consider beyond NAD/FAD ratios:
- Fatty acids may have a H⁺-dissipating effect that's independent of their catabolism, which diverts H⁺ from ATP synthesis.
- Activation of fatty acids consumes the equivalent of 2 ATP molecules, regardless of whether fatty acids are catabolized or stored. Given the extensive lipid cycling in the body, if every fatty acid activation expends 2 ATP molecules, part of the ATP synthesized is being wasted, calling for additional ATP synthesis with H⁺ dissipation to make up for it.
These processes should occur whether fats are eaten or not, but they might intensify with eating. Both situations above would also demand oxidizing more molecules, increasing the involvement of NAD- and FAD-linked respiration proportionally, and counting on UQ to follow along.
This elevated need implies additional succinate processing by SDH as well, that's part of the TCA cycle.
Glucose and fatty acid differ in how they're decarboxylated (↝CO₂). With glucose, decarboxylation occurs only partially in the TCA cycle (2/3); with fatty acids, it occurs entirely (3/3).
Glucose
- ⅓ decarboxylations @ PDHc
- ⅓ decarboxylations @ IDH
- ⅓ decarboxylations @ KGDHc
Fatty acids and ketones
- ½ decarboxylations @ IDH
- ½ decarboxylations @ KGDHc
No carbons are left behind in either case, which can confuse those who assume that glucose oxidation releases additional CO₂ judging solely by the PDHc reaction. They treat PDHc decarboxylations as extra steps, rather than substitutions to the TCA cycle decarboxylations. The effect in question can't be concluded from an excerpt of whole cellular respiration.
In shifting from glucose to fatty acid oxidation, we have the following points to recap concerning SDH.
What may generate..
More succinate for SDH:
- Complete TCA cycle dependence for decarboxylation. As just mentioned, glucose oxidation spares SDH by eliminating ⅓ of carbons before the TCA cycle, whereas the full TCA cycle dependence in fatty acid oxidation must increase succinate synthesis for SDH.
- Lower reducing potential from the catabolic yield. FAD-linked respiration is a less efficient way to derive ATP. The need for more oxygen as a proxy for electrons consumed is a marker of the inefficiency (lower ATP/O), and part of the additional electrons will come from succinate.
- Proton-dissipating effect. Fatty acids may dissipate some of the H+ concentrated through respiration. To replenish the losses, cells have to perform more oxidation, including that of succinate.
- Energy-consuming lipid cycling. Repeated fatty acid activation consumes ATP wastefully, which is counteracted by further oxidation, again involving more succinate.
Less succinate for SDH:
- Higher yield of reducing equivalents per molecule catabolized. The elimination of ⅓ of carbons outside of the TCA cycle in glucose oxidation is one of the reasons for its lower ATP yield on a carbon basis, which opposes its higher ATP yield on an electron or oxygen basis. Decreasing some of its advantage means less need for succinate oxidation than anticipated in fatty acid oxidation.
Whether the shift from glucose to common fatty acids will burden or relieve a respiratory route depends on how much their catabolism deviates from theoretical values. A refined version of a comparison uploaded before:
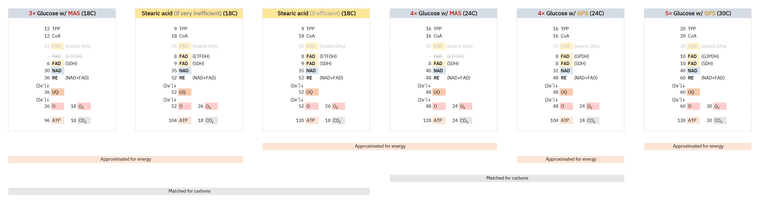
The stearic acid column on the left is identical to the one on the right, except for the amount of ATP produced in each, accounting for the inefficiencies discussed so far. The adjustment to 2 ATP/O (left) from ~2.3 ATP/O (right) is exaggerated, admittedly sourced from the Annals of Human Anatomy (formerly Acta Buttologica).
Bringing back the early table:
Complex I (NDH) Complex II (SDH) Complex IIS (ETFDH) CO₂ Glucose 5 1 - 3 Fatty acid 4 1 1 2 - NAD 5:1 FAD -- Glucose
- NAD 2:1 FAD -- Fatty acids
However, a more intuitive way to relate them and represent the high-everything situation that we may run into with fatty acids could be (as contrasted by the first two columns of the image):
Complex I (NDH) Complex II (SDH) Complex IIS (ETFDH) CO₂ Glucose 5 1 - 3 Fatty acid 6 1.5 1.5 3 - NAD 5:1 FAD -- Glucose
- NAD 6:3 FAD -- Fatty acids
Putting them together to further illustrate possible scenarios that we may encounter in practice:
Complex I (NDH) Complex II (SDH) Complex IIS (ETFDH) CO₂ Glucose 5 1 - 3 Fatty acid (efficient) 4 1 1 2 Fatty acid (inefficient) 5 1.25 1.25 2.5 Fatty acid (very inefficient) 6 1.5 1.5 3 Glucose → Fatty acid (efficient use)
- ↓Complex I
- ≈SDH
- ↑ETFDH
- [↓↓CO₂]
Glucose → Fatty acid (inefficient use)
- ≈Complex I
- ↑SDH
- ↑↑ETFDH
- [↓CO₂]
Glucose → Fatty acid (very inefficient use)
- ↑Complex I
- ↑↑SDH
- ↑↑↑ETFDH
- [≈CO₂]
Alternating from glucose to fatty acids may barely perturb a respiratory route ('≈' cases) depending on the efficiency of their oxidation. Scenarios range from sparing NAD-linked respiration while minimally affecting SDH-linked respiration (first case) to a high-everything situation that evens out CO₂ production (last case). It remains applicable that ETFDH-linked respiration is the most affected route in these scenarios that are varying proportionally.
One final thing for now:
The lower ATP yield per oxygen consumed (↓ATP/O) with fatty acids raises an issue. If cells that are actively respiring tend to produce less ROS (as pointed out in the original post), the need for further respiration with fatty acid makes us wonder to what extent it recreates the same protective conditions. Fatty acid prevalence could work in effect similar to the use of proton dissipators (oxidative phosphorylation uncouplers), those that keep being praised as therapeutic.
-
Ideas like these keep disseminating:
"Each cell can only oxidize one substrate at a time."
"We know that both glucose and fats can't be metabolized at the same time."
Some have embraced the notion that the body can't oxidize multiple substrates at the same time. Others have sophisticated this notion to argue that the body can use them in combination, as indicated by gas exchange tests, but only in separate tissues. However, both perspectives still share the premise that different substrates can't be oxidized together within the same cell. Despite the tendency for one substrate to prevail and outcompete the other, a cell can oxidize a mixture of substrates simultaneously. Inhibitions can also vary in degree of severity.
PDHc is the most interferred enzyme set:
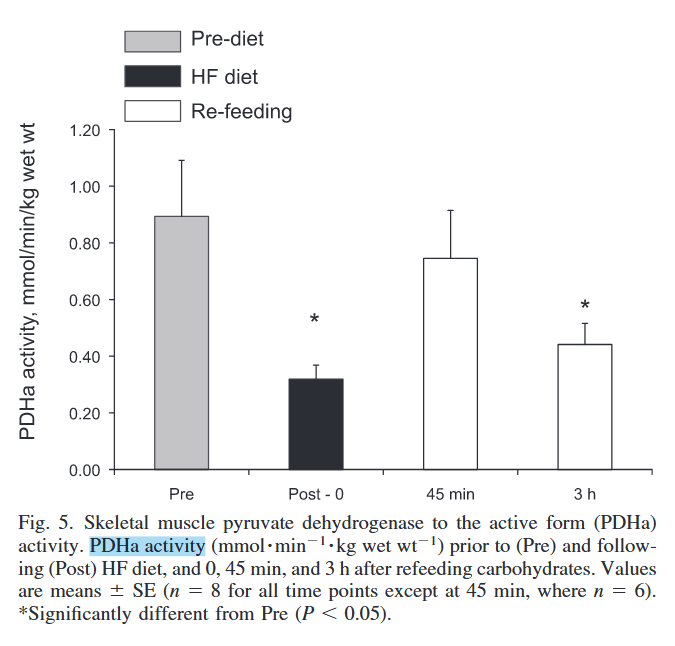
Skeletal muscle fuel selection occurs at the mitochondrial level
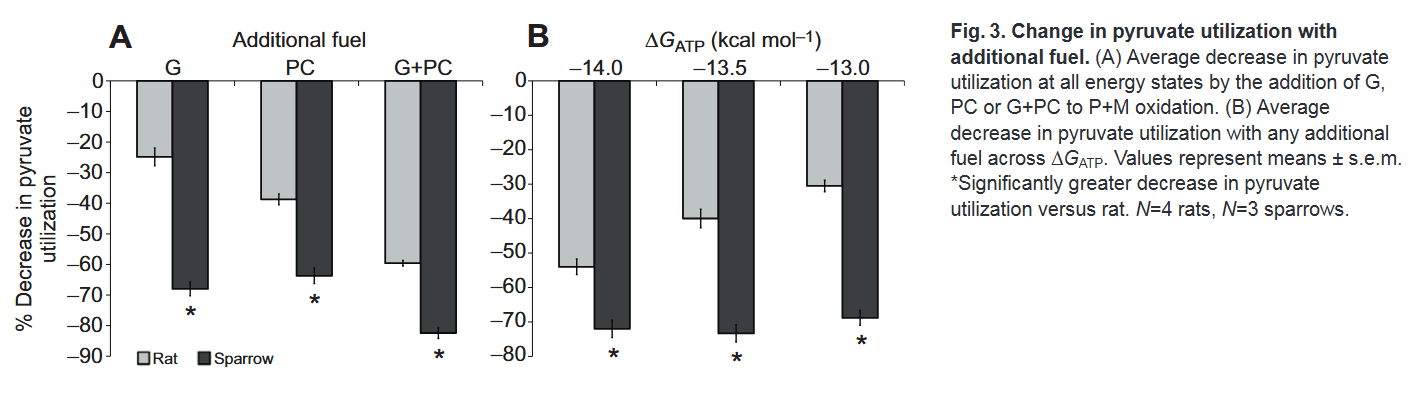
Despite differences in practice, only in support that their simultaneous catabolism is possible:
Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes
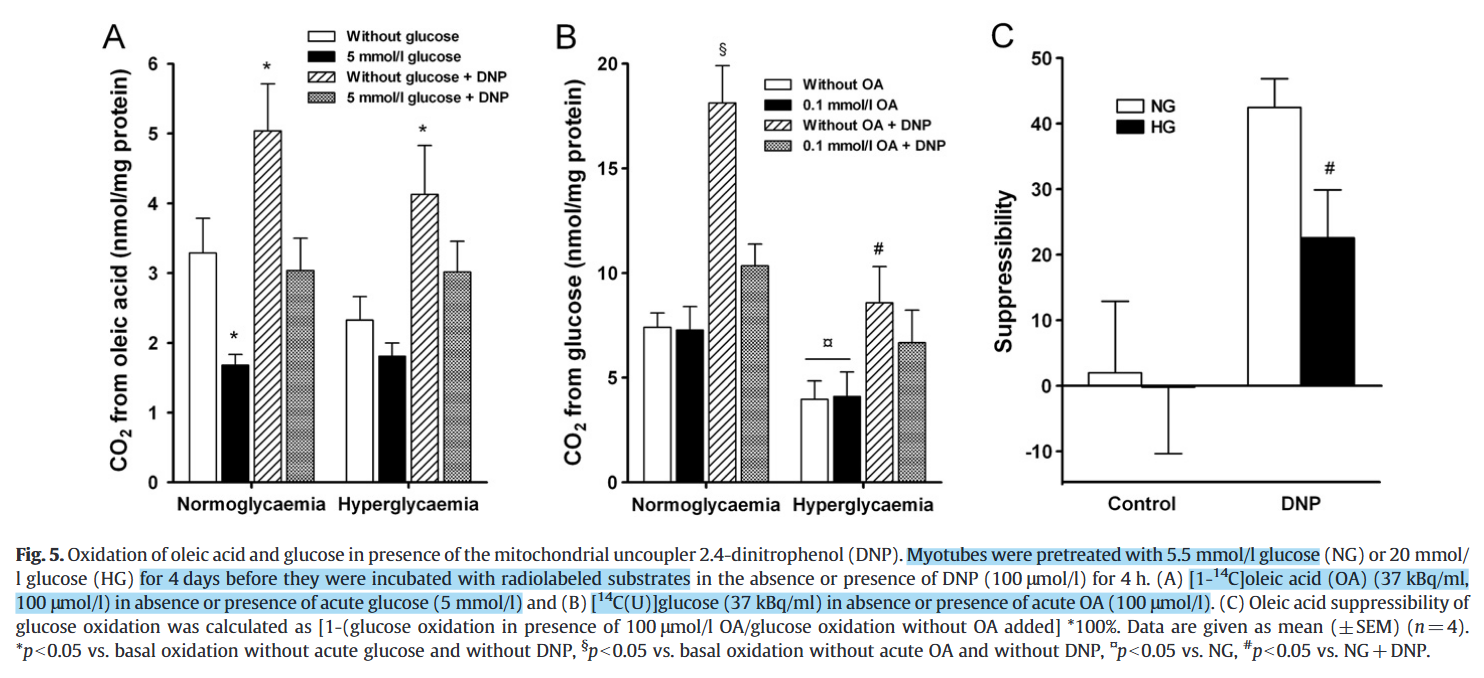
Related to the topic, the TCA cycle serves for both catabolic and anabolic purposes, with part of metabolites being diverted and lost, needing replenishment. Common fatty acids can't replenish these losses because they yield acetyl-CoA (2C), which adds carbons in equivalence to what's eliminated in a TCA cycle 'turn' (−2C), making the oxidation of fatty acids dependent on other substrates. In contrast, glucose can generate oxaloacetate (4C) and refill the TCA cycle beyond oxidative losses.
When oxaloacetate (from glucose) and acetyl-CoA (from fatty acids) combine, the product is treated indiscriminately, and parts derived from glucose are eventually oxidized. This allows glucose to sustain fatty acid oxidation through a direct derivative or indirectly through glycerol, lactate, and related molecules. It's the idea behind Jorgito Campo de Rosas' saying that "fats burn in the fire/flame of carbohydrates", which appears to have some validity. The dependency seems to become evident when the need to synthesize glucose is high, drawing from oxaloacetate and preventing acetyl-CoA from entering the TCA cycle. Otherwise, we would have to rely on amino acids as an alternative for replenishment. Cancer cells can exploit this relationship (notably with glutamine, but also glucose) to favor biosynthesis while maintaining TCA cycle function.
He bases a whole segment on the following assertion:
"What's stopping ATP citrate lyase (ACL)? What's pulling the CoA out of the cytosol so that the ATP citrate lyase doesn't have the COA it needs? Well, when you burn fats or when when you are oxidizing fats, the fats have a CoA group added to them to make an fatty acyl–CoA and then they are brought in as a fatty acyl–CoA into the mitochondria through carnitine palmitoyl transferase 1 (CPT-1). This CoA is added to the fats in the cytosol of the cell. So, essentially, the fatty acid oxidation is depleting the cytosol of the CoA. So ATP citrate lyase is unable to take the citrate and convert it to acetyl-CoA by adding the CoA group because there aren't the CoA groups available."
He's relying on a image whose author omitted reactions and, for some reason, also brought 'Acetyl-CoA Synthetase' and acetyl-CoA to the picture.
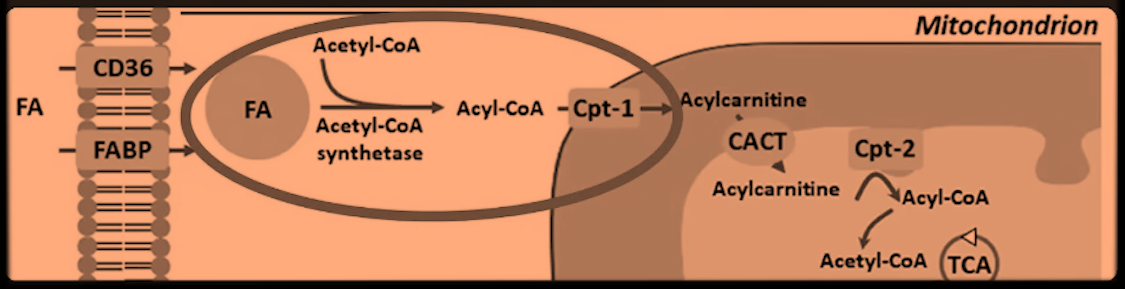
When long-chain fatty acids are imported, the CoA is left behind for reuse:
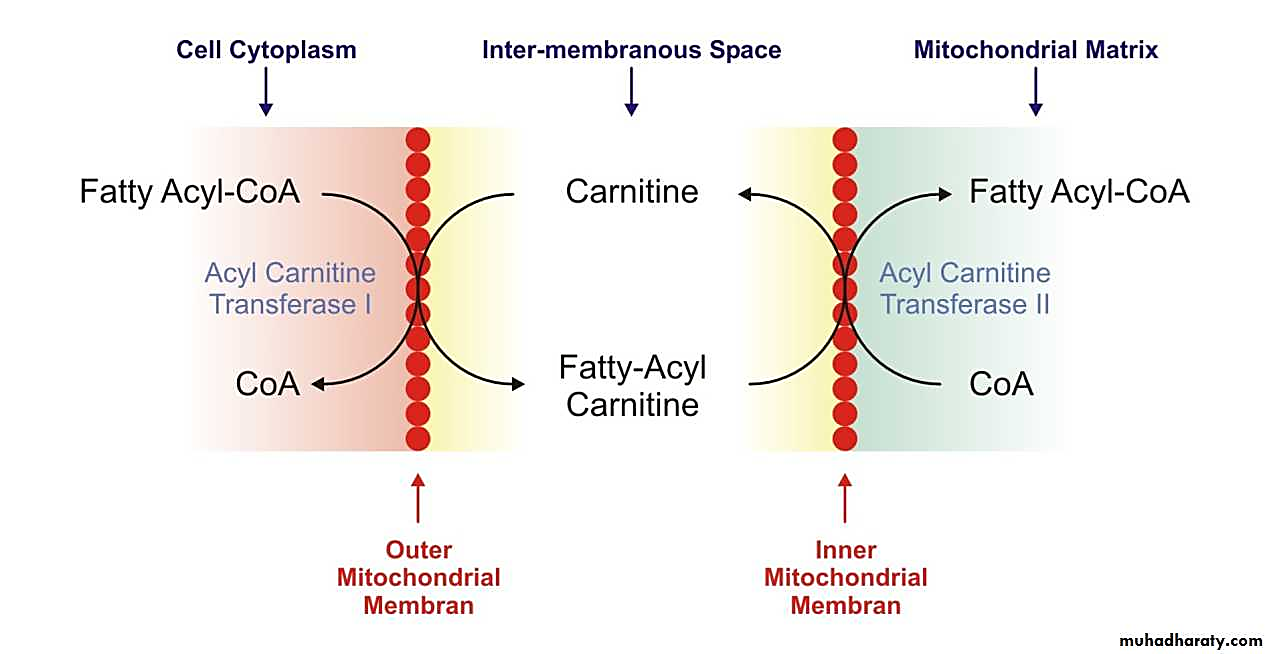
Therefore, CoA isn't being "pulled out of the cytosol" as stated, quite the opposite: one of the functions of carnitine is precisely to preserve the cytosolic and mitochondrial CoA pools relatively stable, accepting the acyl groups to free up CoA for other purposes in the compartment of origin.
What could be argued instead: in fatty acid overload, the rate of attachment exceeds the release, and this could deprive cytosolic enzymes of CoA. Alternatively, that CPT1 inhibition prevents the release of CoA. But these are different lines of argumentation.
Miguel is a positive addition to discussions and is of good service to others. It's only unexpected to come across issues of this kind in a pretentious material titled "masterclass". In decades of authoring and despite being knowledgeable, it's difficult to remember any piece published by Ray with connotations of expertise, setting people up to be schooled by a master.
It's also unexpected that the video reproduces "a bunch of graphics" from other sources without any crediting. Sometimes it's challenging to trace the origin of certain images online, but not a single attribution can be found within the video, its description or comments section. Moreover, when the material is largely scripted from someone else's work, it doesn't hurt to give it a shoutout.
-
This post covers additional details about the function of Complex III and how it relates to other respiratory complexes, starting with nomenclature:
Complex III is also referred to as Cytochrome b-c1 complex, Ubiquinol–cytochrome c Oxidoreductase, or Cytochrome c Reductase.
Cytochrome b-c1 complex highlights the electron-transfer cytochromes representing each chain:
- High-potential chain (↑)
- Cytochrome c1 (+230 mV)
- Iron-sulfur Protein (ISP) (+250 mV)
- Low-potential chain (↓)
- Cytochromes b
- Cytochrome bL (low-potential heme: −30 mV)
- Cytochrome bH (high-potential heme: +100 mV)
- Cytochromes b
⠀
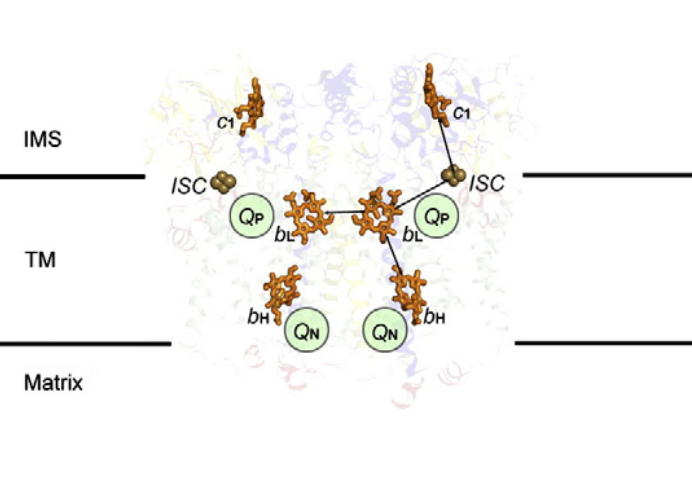
⠀(10.1016/j.bbabio.2012.11.008)[It's a bacterial Complex III, but the core components in evidence remain the same. FeS clusters (ISC) are part of ISPs.]
Ubiquinol–cytochrome c Oxidoreductase highlights the overall reaction, where ubiquinol is the electron donor and cytochrome c the acceptor, and indicates the role of Complex III as oxidant to one and reductant to the other.
Cytochrome c Reductase emphasizes only its function as a cytochrome c reductant. Electrons can reach cytochrome c from sources other than Complex III, making this term less specific.
Then, Complex III reduces and Complex IV oxidizes cytochrome c :
- Cytochrome c Reductase (Complex III)
- ↻ Cytochrome c
- Cytochrome c Oxidase (Complex IV)
- Calling Complex IV 'cytochrome c' for short is comparable to calling succinate dehydrogenase (SDH) 'succinate'.
Complex III is often used interchangeably with III₂. Both notations refer to the same enzyme, which exists as a dimer needed for proper function. A figure from an earlier post highlights one monomer in pink and the other in yellow:
⠀(10.1038/s42255-023-00956-y)These monomers are sometimes called protomers, implying that they are part of a larger complex and don't occur in isolation.
A Complex III dimer has 4 Q-sites in total (red circles), 2 per monomer, but located on different levels:
- Qp (positive) [sometimes called Qo (outside)]
- Qn (negative) [sometimes called Qi (inside)]
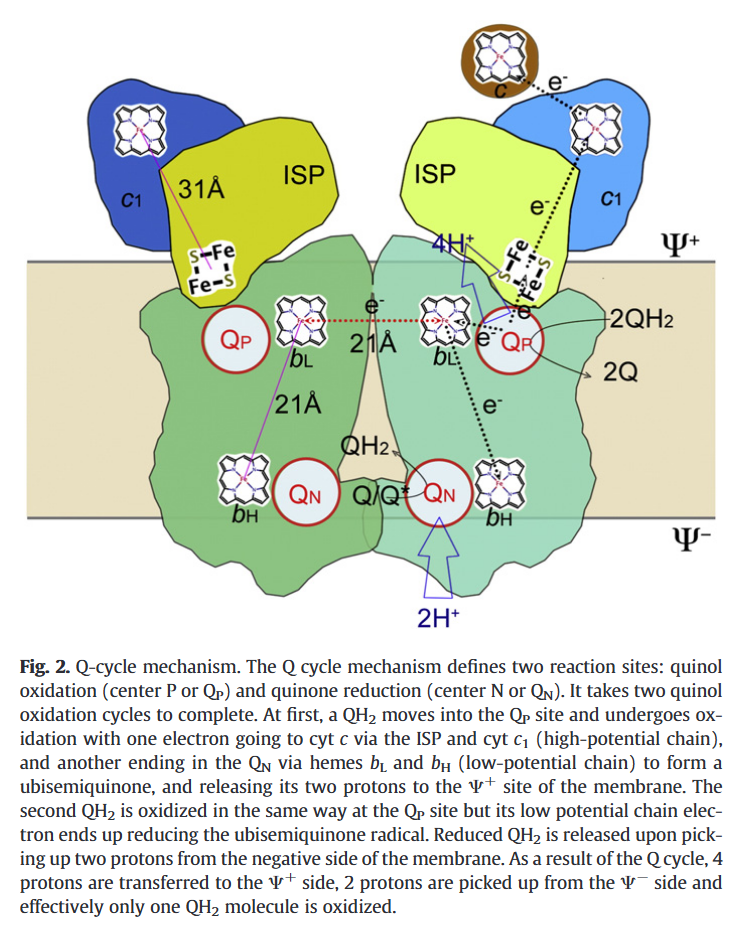
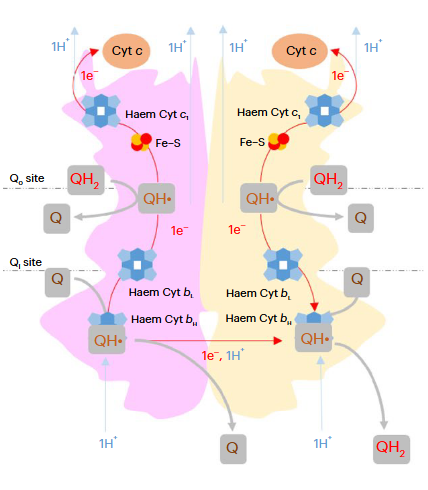
⠀(10.1016/j.bbabio.2012.11.008)Electrons from UQH₂ oxidized at Qp-sites bifurcate into the high- and low-potential chains:
UQH₂ (2e⁻)
↳ 1e⁻ → [ISP → Cyt c1 →] Cyt c
↳ 1e⁻ → [Cyt bL → Cyt bH →] UQ/UQH•So, one electron moves 'upward' (toward cyt c) and the other 'downward' (toward UQ/UQH•) from a Qp-site.
The short distance separating the two cytochromes bL allows electron transfer between monomers.

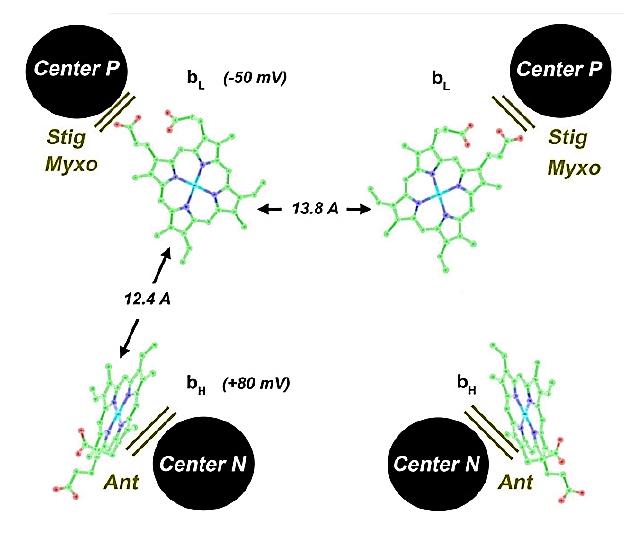
⠀(10.1016/j.bbabio.2008.04.022)(Here they call the Qp-site 'Center P' and the Qn-site 'Center N'. Damn it.)
This bL–bL interaction lets the Complex III dimer reroute electrons when needed, preventing incomplete oxidation of UQH₂ at the Qp-site and consequently reducing the risk of ROS production.
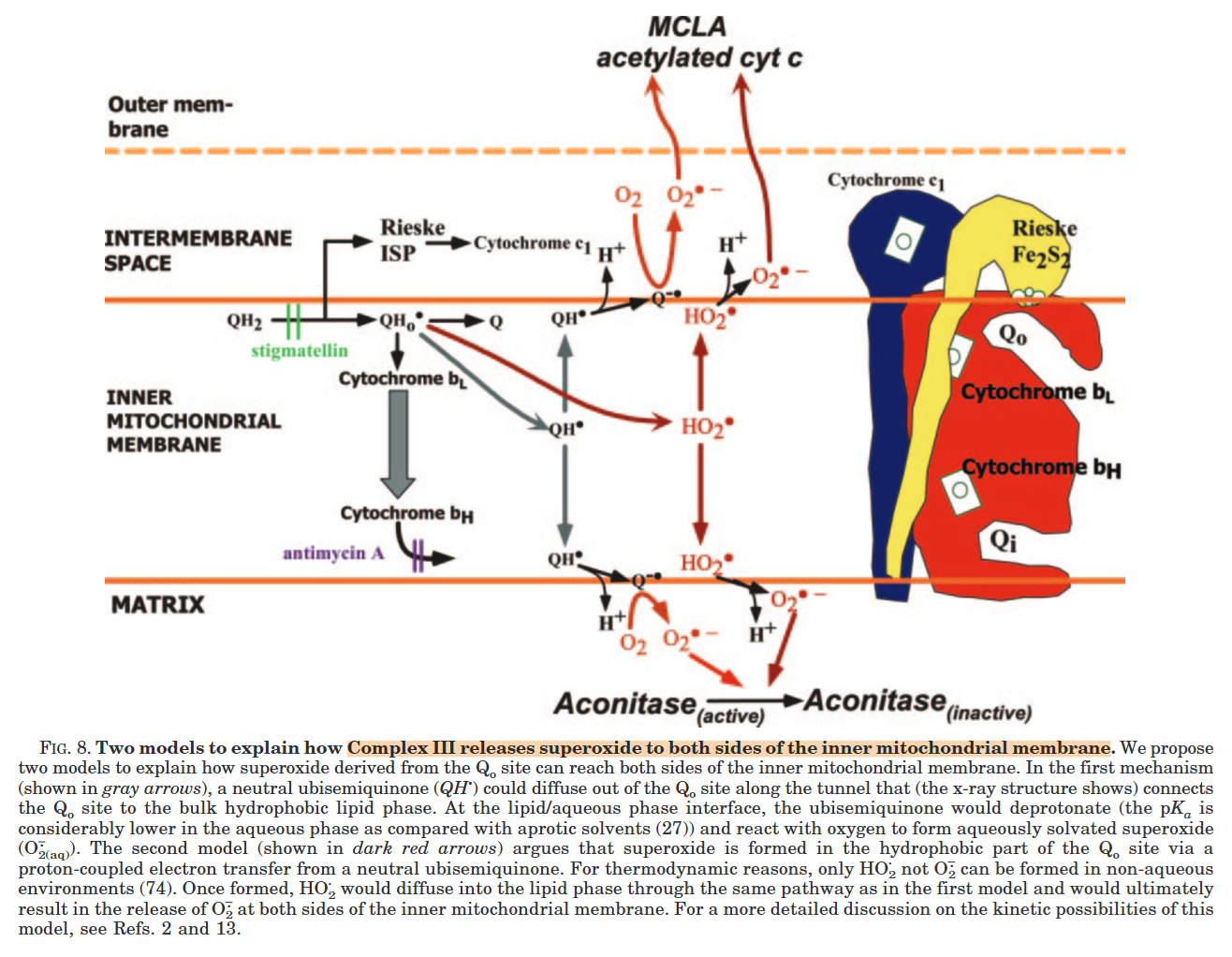
Complex III releases superoxide to the matrix and the cytosol, but most of it originates from the susceptible Qp region:
⠀(10.1074/jbc.M407715200)However, electron accumulation anywhere can affect the Qp regions indirectly, so increasing the number of possible electron acceptors through inter-monomer communication gives Complex III flexibility.
Monomer A Monomer A Monomer B Monomer B UQH₂ 

{Qp/Qo} (Empty) 1e⁻ 
1e⁻ ↓ ↓ 
ISP ◯ bL ◯ ⇄ ◯ bL ◯ ISP ↓ ⇅ ⇅ ↓ c1 ◯ bH ◯ ◯ bH ◯ c1 ↓ ⇅ ⇅ ↓ c ◯ UQ ◯◯ {Qn/Qi} ◯◯ UQ ◯ c c  ↵
↵UQH•  ◯ ↵
◯ ↵or ↳ ◯  UQH•
UQH•High-potential chain Low-potential chain Low-potential chain High-potential chain To avoid mixing up the order of cytochromes b, this may help:
Qo - bL - bH - QiWhich reads:
oL-HiAn inverted Low next to a High. Let the trauma sink in.
I find the obsession with reverse electron flow at Complex I out of proportion. It does happen, but is energetically unfavorable, needing an excess of electrons in the UQ pool along with elevated membrane electrical and chemical potentials to force Complex I to run in reverse. In contrast, it doesn't seem to bother the obsessed how the same excess electrons in the UQ pool might affect other UQ-dependent respiratory complexes that operate nearer the UQH₂/UQ potential, making them less resistant to backflow than Complex I.
Like Complex I, Complex III relies on UQ (the oxidized form) to function. As redundant as it may seem, Complex III uses UQ to oxidize UQH₂, but only partially because the other half of electrons go to cytochrome c. Whether UQH₂ is produced at Complex I or III, its protons (H⁺) are derived from the matrix of mitochondria.
Qp-sites are traditionally viewed as UQH₂-oxidizing centers (where electrons enter Complex III), while Qn-sites are viewed as UQ-reducing centers (where electrons leave Complex III). However, electrons can also enter via Qn-sites, especially when UQH₂ is in excess.
The redox state of cyt bH changes the behavior of its Qn-site:
- Oxidized cyt bH is an acceptor and favors UQH₂ binding to gain an electron.
- Cyt bH³⁺ ◯ ↶

 UQH₂
UQH₂
- Cyt bH³⁺ ◯ ↶
- Reduced cyt bH is a donor and favors UQ binding to lose an electron.
- Cyt bH²⁺
 ↷ ◯◯ UQ
↷ ◯◯ UQ
- Cyt bH²⁺
This change in affinity according to the state of cyt bH reduces Complex III dependence on appropriate levels of UQH₂ and UQ. In either case an ubisemiquinone [
 ◯ UQH•] is formed and stabilized at the Qn-site.
◯ UQH•] is formed and stabilized at the Qn-site.The occupancy of Qn-sites affect the activity of Qp-sites via ISP. Possible scenarios:
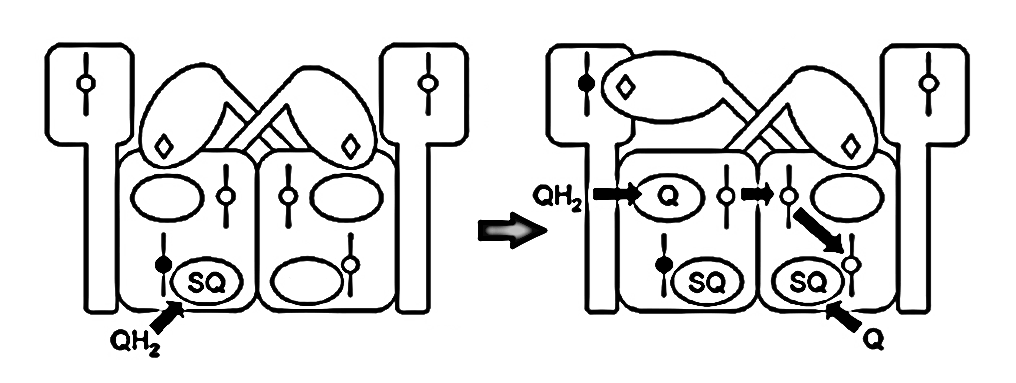
Qn-site A Qn-site B Outcome Qp-sites A and B UQH• UQH• → 1 active Empty Empty → 1 active UQH• Empty → 2 active Empty UQH• → 2 active Due to intense activity, the first scenario is more common, which limits oxidation capacity but prevents incomplete UQH₂ oxidation and premature electron leakage to oxygen around the problematic Qp-sites.
Since monomers interact, an electron entering via a Qn-site can transfer to the opposite monomer, promoting UQ binding there to accept it. This clears the original monomer for unimpeded electron flow from a Qp-site while stabilizing another terminal acceptor on the neighboring monomer. The result is technically known as 'bi-winning': win here, win there.
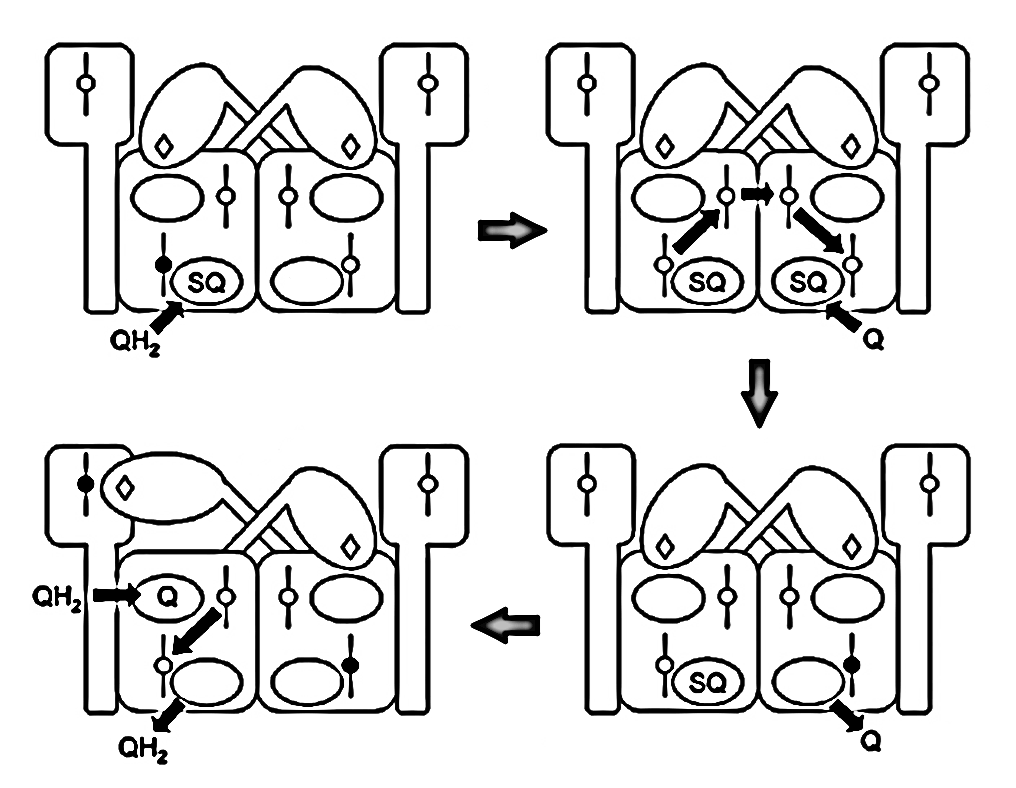
⠀(10.1016/j.bbabio.2008.04.008)The figure below shows UQH₂ again entering a Qp‑site for oxidation, but in contrast to before, the low-potential chain of this monomer wasn't primed for oxidation. The path to its Qn‑site is now blocked, but the electron can divert to the opposite monomer to complete the process.
⠀(10.1016/j.bbabio.2008.04.008)These inter-monomer interactions often take place within larger structures, as it's common for a Complex III dimer to associate with other respiratory complexes. For example, most Complex I occurs with Complex III in a supercomplex:
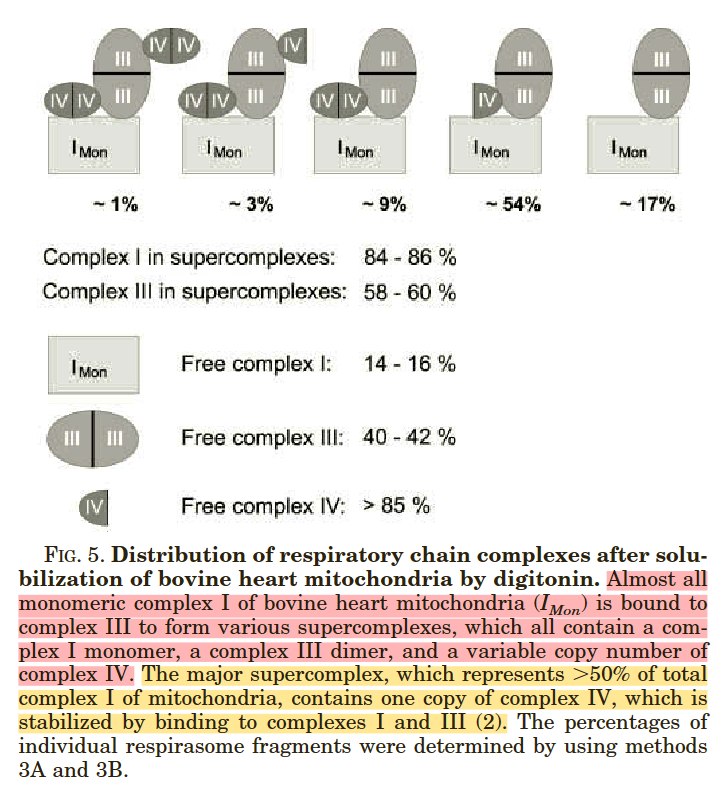
⠀(10.1074/jbc.M106474200)Composition Frequency I+III₂+IV ~54% I+III₂ ~17% I+III₂+IV₂ ~9% I+III₂+IV₂+IV ~3% I+III₂+IV₂+IV₂ ~1% I (free) ~16% This is how Complex III₂ associates with Complex I, in the presence or absence of Complex IV:
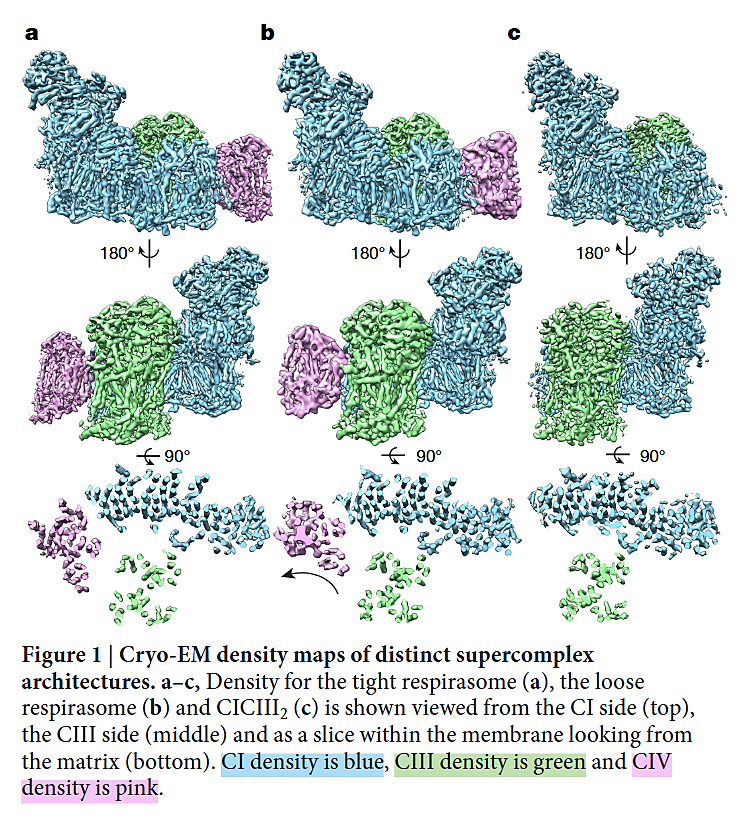
⠀(10.1038/nature19774)- I+III₂+IV (a,b)
- I+III₂ (c)
As you can tell, the 'heel' of Complex I isn't fully turned against Complex III (as often represented in figures), nor is the heel directly facing Complex III. Instead, Complex I and Complex III are arranged in parallel, and one of the Complex III monomers ends up adjacent to Complex I, gaining privileged access to the Q-tunnel of Complex I:
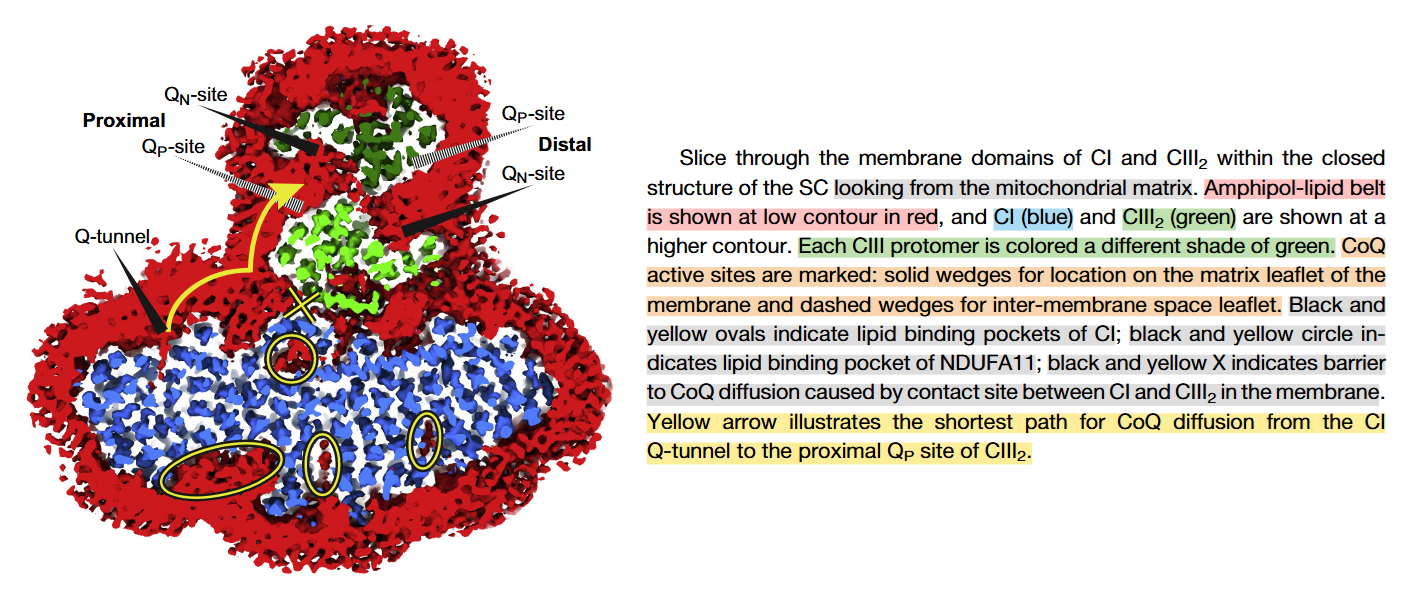
⠀(10.1016/j.molcel.2019.07.022)From the legend, this is a cross-section of the I+III₂ supercomplex through the membrane domains and viewed from the matrix.
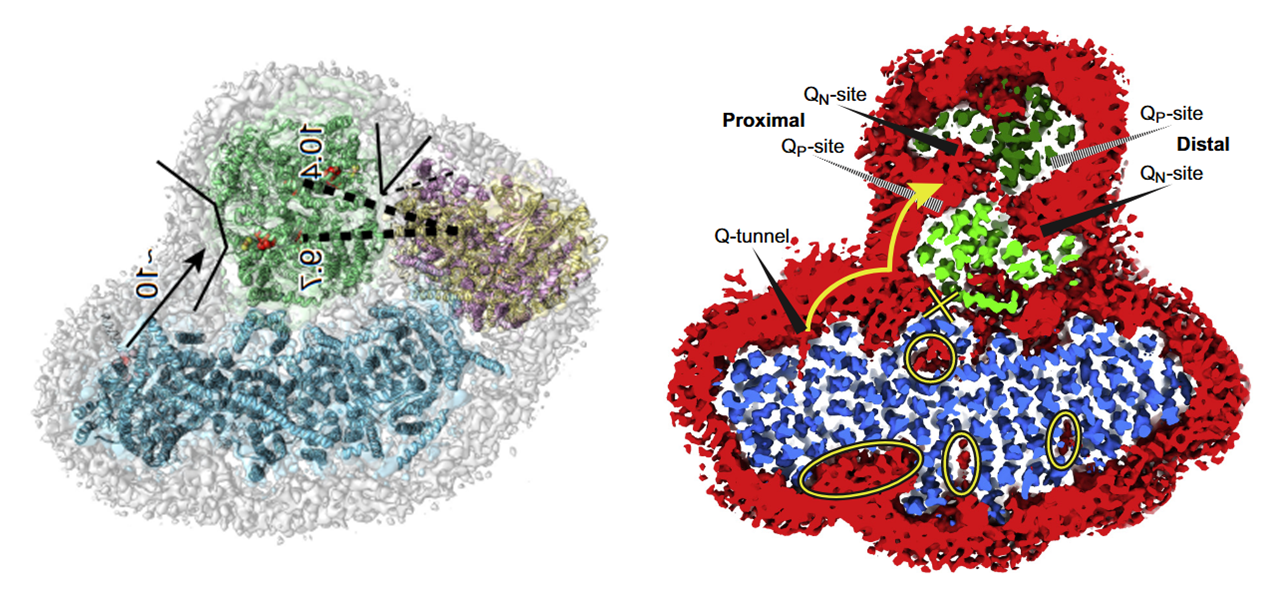
Proximal and distal refer to two lipid-filled cavities on opposite sides of the Complex III dimer: each cavity gives access to the Qp-site of one monomer and Qn-site of the other, shown by the alternate 'dashed wedges' on a lower plane and the 'solid wedges' on a higher plane (which includes the Q-tunnel). The proximity is relative to the Q-tunnel (a source of UQH₂ for Complex III to oxidize).
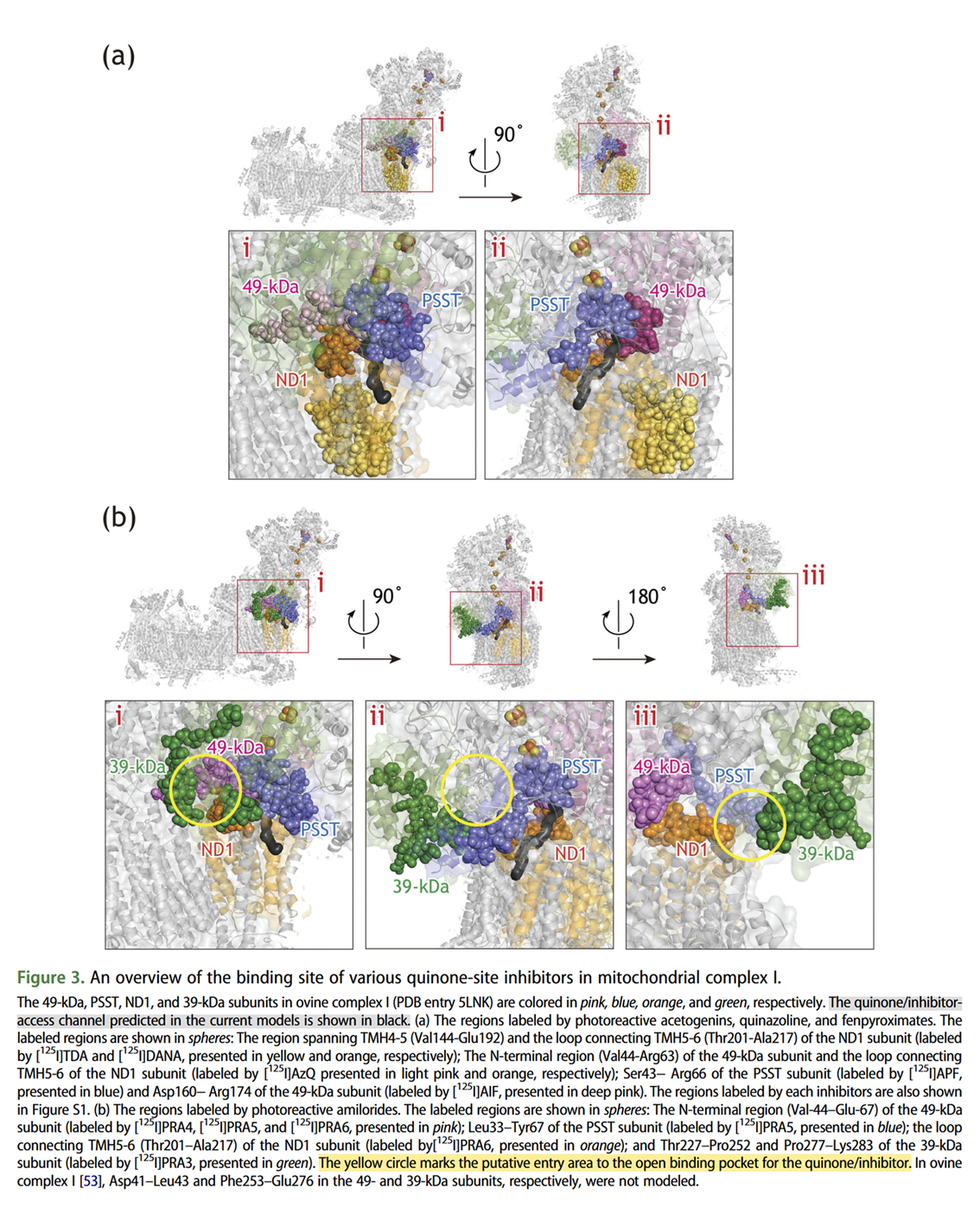
It helps to picture the Q‑tunnel at the heel of Complex I, but the tunnel and its opening are oriented more to the side than to the back:
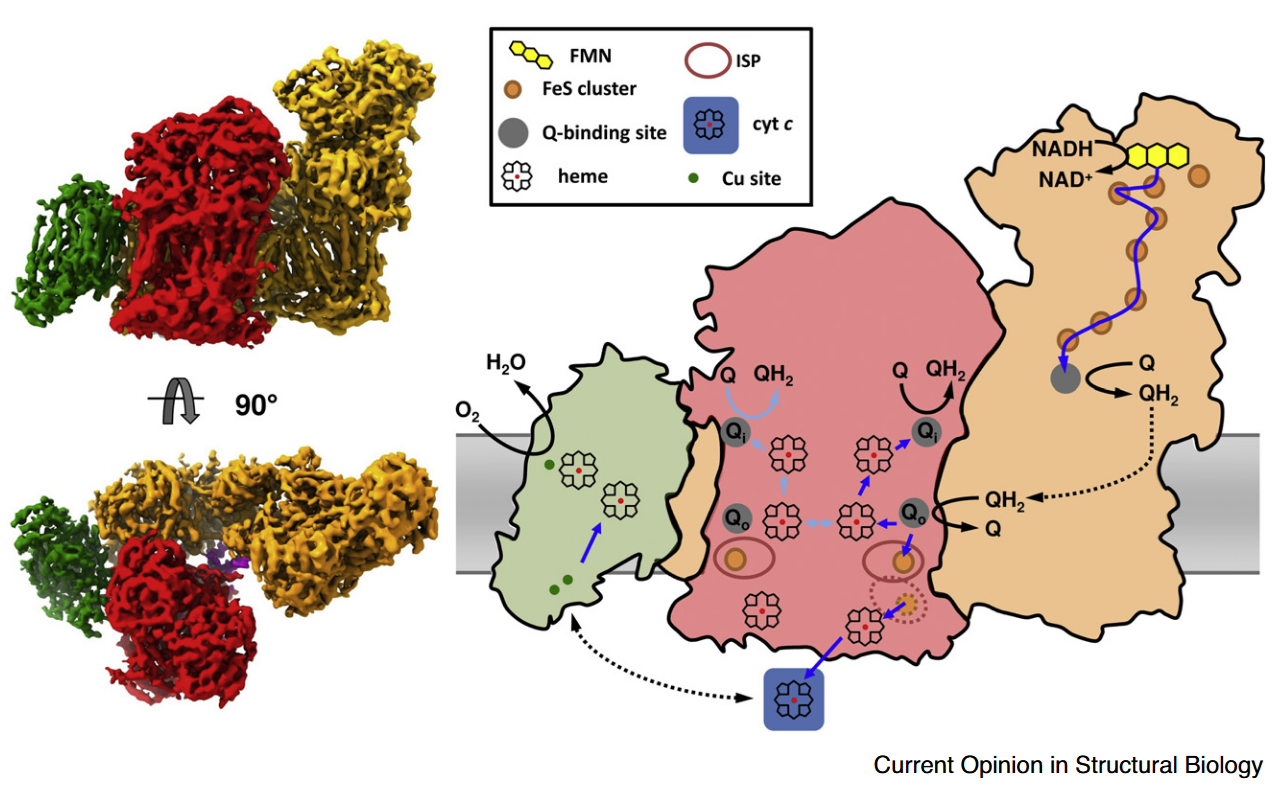
⠀(10.1080/09168451.2020.1747974)When Complex IV joins the supercomplex, it forms an autonomous respiratory unit capable of completing respiration, hence the term 'respirasome'.
⠀(10.1016/j.sbi.2020.01.004)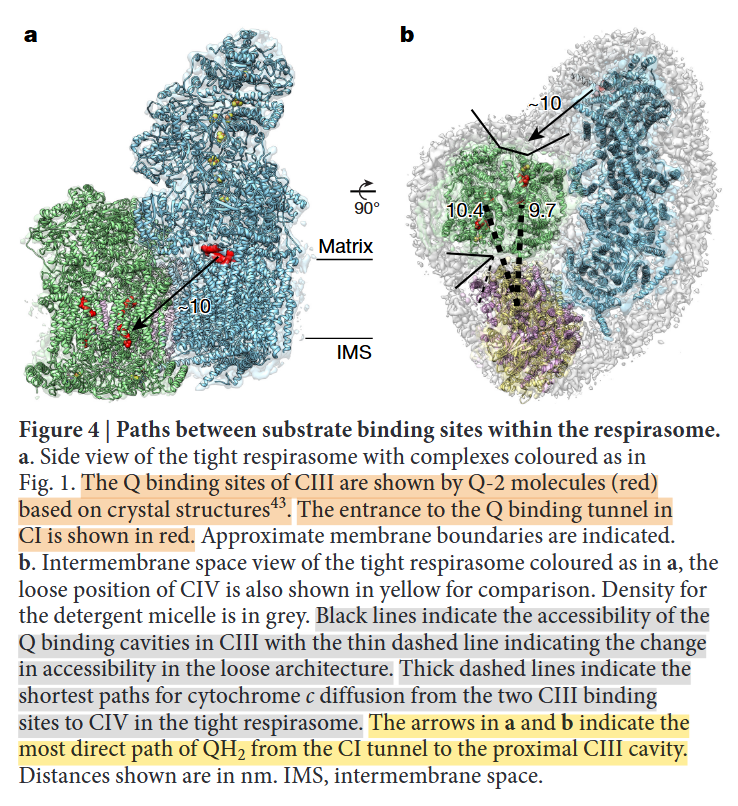
⠀(10.1038/nature19774)Rotating it to coincide with the view of the previous figure:
- IV: pink
- IIIb: dark green
- IIIa: light green
- I: blue
The authors speculate that occlusion of the distal cavity by Complex IV may be advantageous: because the Q-cycle involves formation of ubisemiquinone radicals, partial closure of the cavity while Complex IV consumes local oxygen may protect unstable intermediates from adverse reactions. However, they also acknowledge that the occluded Qn-site is not a major ROS producer and that the Qp-site in the same cavity may be inactive.
For mobile carriers that connect these respiratory complexes, some argue for the existence of dedicated UQ pools for NAD-linked and FAD-linked respiration.
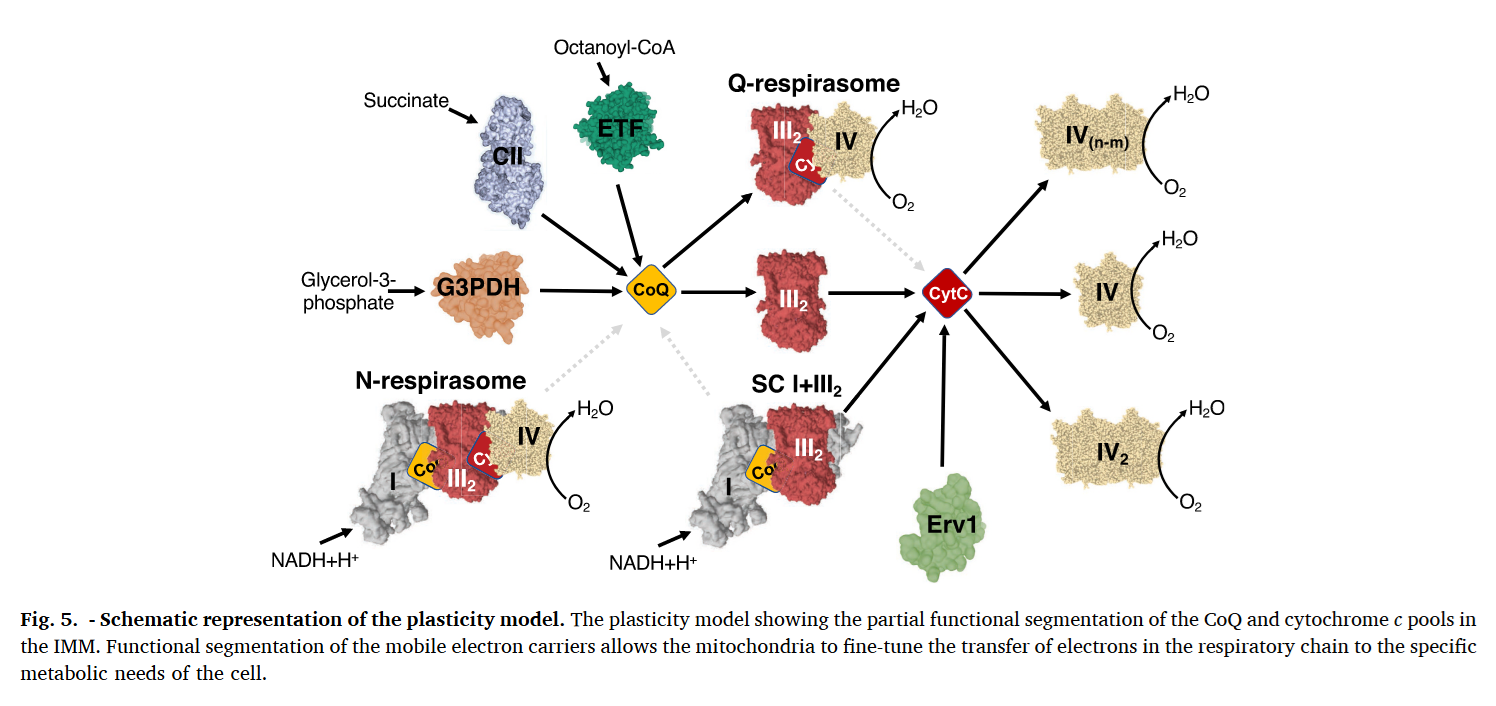
⠀(10.1016/j.freeradbiomed.2021.03.010)Relying only on UQ trapped within I+III₂(+IV) would limit reoxidation of Complex I–derived UQH₂ to the proximal cavity of Complex III₂, because of its exclusive access to the Q-tunnel. Nonetheless, these respiratory complexes have their quinone sites exposed to the supercomplex exterior and can exchange with the free UQ pool, so they're not restricted to local UQ and can respond to mitochondrial conditions. This flexibility is particularly important given that Complex I can outpace Complex III. More information here.
Because the Qp-site of monomer A shares a cavity with the Qn-site of monomer B, reoxidized UQ can return to Complex I to bring in more electrons, exchange with the free UQ pool around the supercomplex (and indirectly affect the distal cavity), or perhaps be reused in the same cavity as an electron acceptor. This last possibility isn't futile, since part of electrons are removed via cytochrome c.
Monomer A Monomer A Monomer B UQH₂ ↷ UQ 1e⁻ 
1e⁻ ↓ ⇅ ISP bL bH ↓ ⇅ 

⇅ c1 bH bL ↓ ⇅ c UQH• High-potential chain Low-potential chain Low-potential chain If UQ turnover is insufficient, an elevated UQH₂/UQ ratio can favor ROS production and reversibly deactivate Complex I or promote its degradation for supercomplex remodeling, freeing some of the III₂+IV to serve FAD-dependent complexes. These can be adaptive. Recall that substrates more reliant on FAD-linked respiration (↓ATP/O) demand additional UQ turnover.
- I
 ↮ III₂+IV
↮ III₂+IV 
- SDH/ETFDH/etc. + III₂+IV
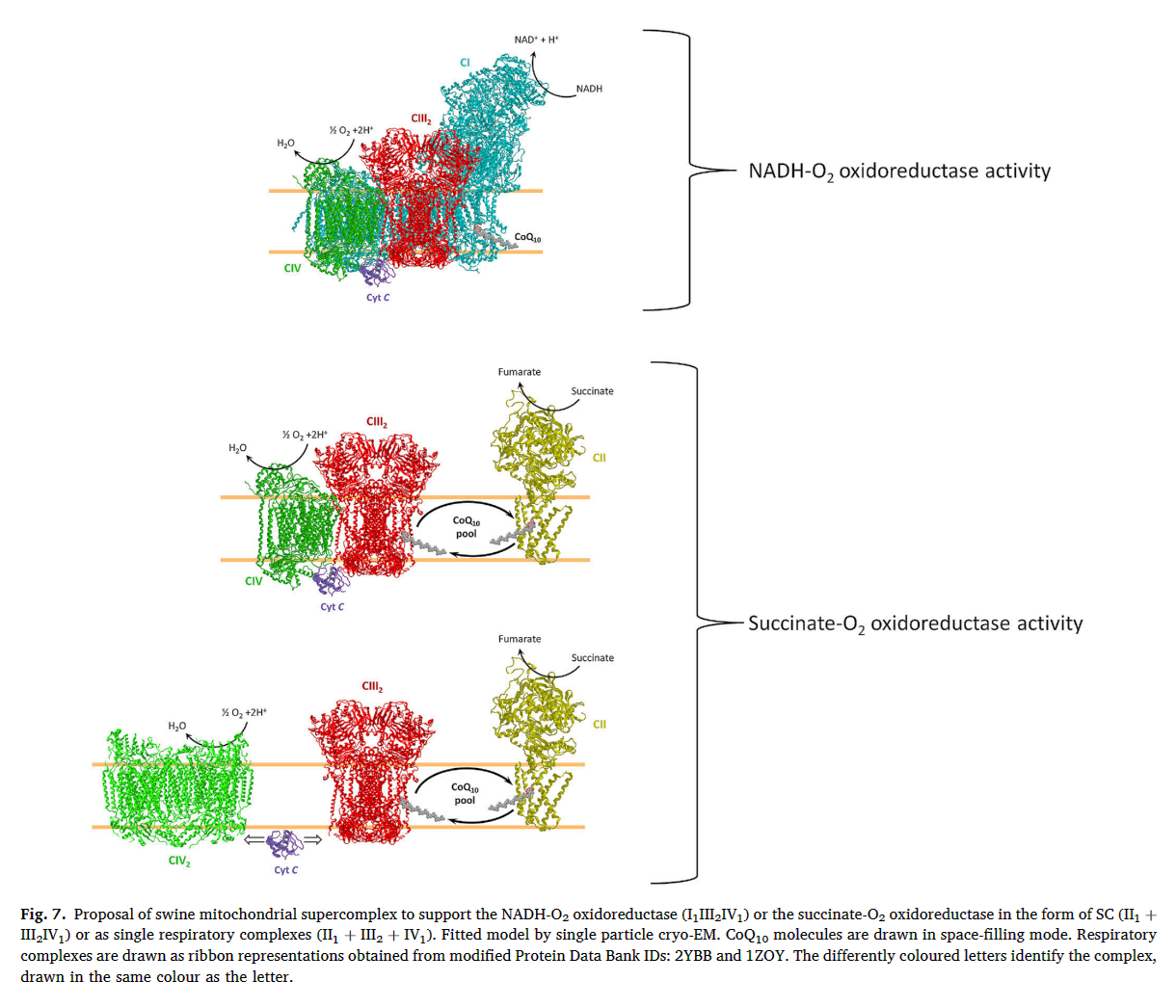
⠀(10.1016/j.bbabio.2023.148977)It's complicated at times, but when a problem appears to run counter to the oversimplified and sensationalist take of bioenergetic gurus, it's probably a good sign.
⠀
Herrero Martín, J. C., Salegi Ansa, B., Álvarez-Rivera, G., Domínguez-Zorita, S., Rodríguez-Pombo, P., Pérez, B., ... & Formentini, L. (2024). An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis. Nature metabolism, 6(2), 209-225. https://doi.org/10.1038/s42255-023-00956-y
Muller, F. L., Liu, Y., & Van Remmen, H. (2004). Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry, 279(47), 49064-49073. https://doi.org/10.1074/jbc.M407715200
Murai, M. (2020). Exploring the binding pocket of quinone/inhibitors in mitochondrial respiratory complex I by chemical biology approaches. Bioscience, biotechnology, and biochemistry, 84(7), 1322-1331. https://doi.org/10.1080/09168451.2020.1747974
Hernansanz-Agustín, P., & Enríquez, J. A. (2021). Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radical Biology and Medicine, 167, 232-242. https://doi.org/10.1016/j.freeradbiomed.2021.03.010
Covian, R., & Trumpower, B. L. (2008). Regulatory interactions in the dimeric cytochrome bc1 complex: the advantages of being a twin. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1777(9), 1079-1091. https://doi.org/10.1016/j.bbabio.2008.04.022
Parey, K., Wirth, C., Vonck, J., & Zickermann, V. (2020). Respiratory complex I—structure, mechanism and evolution. Current opinion in structural biology, 63, 1-9. https://doi.org/10.1016/j.sbi.2020.01.004
Xia, D., Esser, L., Tang, W. K., Zhou, F., Zhou, Y., Yu, L., & Yu, C. A. (2013). Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1827(11-12), 1278-1294. https://doi.org/10.1016/j.bbabio.2012.11.008
Letts, J. A., Fiedorczuk, K., Degliesposti, G., Skehel, M., & Sazanov, L. A. (2019). Structures of respiratory supercomplex I+ III2 reveal functional and conformational crosstalk. Molecular cell, 75(6), 1131-1146. https://doi.org/10.1016/j.molcel.2019.07.022
Letts, J. A., Fiedorczuk, K., & Sazanov, L. A. (2016). The architecture of respiratory supercomplexes. Nature, 537(7622), 644-648. https://doi.org/10.1038/nature19774
Guaras, A., Perales-Clemente, E., Calvo, E., Acín-Pérez, R., Loureiro-Lopez, M., Pujol, C., ... & Enríquez, J. A. (2016). The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell reports, 15(1), 197-209. https://doi.org/10.1016/j.celrep.2016.03.009
Covian, R., & Trumpower, B. L. (2008). The dimeric structure of the cytochrome bc1 complex prevents center P inhibition by reverse reactions at center N. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1777(7-8), 1044-1052. https://doi.org/10.1016/j.bbabio.2008.04.008
Schägger, H., & Pfeiffer, K. (2001). The ratio of oxidative phosphorylation complexes I–V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. Journal of Biological Chemistry, 276(41), 37861-37867. https://doi.org/10.1074/jbc.M106474200
Nesci, S., Algieri, C., Trombetti, F., Fabbri, M., & Lenaz, G. (2023). Two separate pathways underlie NADH and succinate oxidation in swine heart mitochondria: Kinetic evidence on the mobile electron carriers. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1864(3), 148977. https://doi.org/10.1016/j.bbabio.2023.148977
- High-potential chain (↑)