Should 2,6-Dihydroxybenzoic acid be taken with food?
-
Since it is lipophilic and acidic.
I'm not sure if lipophilic = fat soluble though. Usually fat soluble vitamins are better absorbed with food.
Either my mom can take it on an empty stomach, or with food. Or even just mix it with a little saturated fat.
If you're wondering what 2,6 is, it's the metabolite of Aspirin that Georgi mentions in the Strong Sistas podcast as being potentially curative for cancer.
-
@TemetNosce how did you even get that stuff ?
-
@Mauritio Online chemical vendors sell it. Some sell to non business in small quantities. As long as it is not restricted due it being dangerous or some medicine on receipt, you can just buy it. Where do you live? I can buy this legally from a professional party in the Netherlands.
-
@Joop3000 Yeah but you can't USP grade or any grade that is safe for human consumption. They can sell you 99.9% 2,6-Dihydroxybenzoic acid and the 0.1% impurity could be made up of 100%lead and it would be legal because it is expilcetly sold pure research purposes.
-
@alfredoolivas I get where you're coming from, but because of its similarity to aspirin, isn't it safe to assume that the chemists began their synthesis with the widely available acetylsalicylic acid? From doing a little looking into it, all they would need to do is to dissolve the acetylsalicylate in water, which will hydrolyze the compound to acetate + salicylate. Then, the salicylate needs to be oxidized (1 step) to form 2,6 DHBA. Possible oxidizing agents are: potassium permanganate and sodium dichromate. Neither manganese, sodium, potassium, nor chromium are toxic in very low quantities for humans.
So, from my POV is unlikely to be high in heavy metals.
-
@alfredoolivas tell seller that presence of common heavy metals like lead will interfere with the cell culture study results
-
@Joop3000 Oh nice . I live in Germany . Can you send the link ?
-
@jamezb46 said in Should 2,6-Dihydroxybenzoic acid be taken with food?:
Possible oxidizing agents are: potassium permanganate and sodium dichromate. Neither manganese, sodium, potassium, nor chromium are toxic in very low quantities for humans.
How do you know these oxidising agents will be used? Acetylsalicylic acid will also be derived from a source that is for research purposes, I don't see how USP grade being available means they would use it.
If the dihydroxybenzoic acid was being used in doses of a few mgs, the amount of potential risk from impurities would be very low, but if you are taking multiple hundred milligram doses, I would be worried for the impurities as you would potentially be taking impurities.
For example, taking 300mg of 97% powder would expose you to 9mg of impurities a day.
-
@jamezb46 This is the way to go indeed. Check how they synthesize, purify, etc and what is the source and added substances.
It is important to think about these things, we know how even minor impurities can be problematic.
-
I have asked grok for the production process and then t does seem pretty strength forward, not involving too many steps.
Also the impurities are most likely derivatives .
From what I've read the bulk of impurities would consist of resorcinol, which can have anti thyroid effects but at dosages at least a 100 times higher than what we would ingest.
https://pubmed.ncbi.nlm.nih.gov/36693459/It's 25g for like 130€ which is a pretty steep price so of someone in Europe wants to share it, let me know . The company below does indeed seem to ship to private users.
https://www.laboratoriumdiscounter.nl/de/26-dihydroxybenzoic-acid-980gct-25g.html
Grok Conversation:
"Producing 2,6-dihydroxybenzoic acid (also known as gamma-resorcylic acid) in a laboratory typically involves organic synthesis methods, often starting from readily available precursors. Here’s a general outline of how a lab might synthesize it, followed by an explanation of what the remaining 2% in a 98% pure sample might consist of.
Synthesis of 2,6-Dihydroxybenzoic Acid
A common approach to synthesize 2,6-dihydroxybenzoic acid could involve the following steps:
-
Starting Material: A typical starting point might be resorcinol (1,3-dihydroxybenzene), which already has two hydroxyl groups on the benzene ring. The challenge is introducing the carboxylic acid group at the 2-position.
-
Carboxylation: One established method is the Kolbe-Schmitt reaction, a carboxylation process:
- Resorcinol is reacted with carbon dioxide (CO₂) under high pressure and temperature in the presence of a strong base (e.g., sodium hydroxide or potassium hydroxide).
- The reaction proceeds via the formation of a phenolate ion, which attacks CO₂, leading to carboxylation. For 2,6-dihydroxybenzoic acid, the reaction conditions (e.g., temperature, base strength) must favor substitution at the 2-position ortho to one of the hydroxyl groups.
- After acidification (e.g., with HCl), the free acid form of 2,6-dihydroxybenzoic acid is obtained.
-
Purification: The crude product is purified via recrystallization (e.g., from water or an ethanol-water mixture) or column chromatography to remove byproducts and unreacted starting materials, aiming for high purity (like the 98% mentioned).
Alternative routes might include:
- Oxidation of a precursor like 2,6-dihydroxybenzaldehyde or 2,6-dihydroxytoluene, though these are less common and require additional steps to prepare the starting material.
- Enzymatic or microbial synthesis in specialized labs, though this is more research-oriented than standard.
What’s in the Other 2% of a 98% Pure Sample?
If a lab offers 2,6-dihydroxybenzoic acid at 98% purity, the remaining 2% likely consists of impurities from the synthesis process. These could include:
-
Unreacted Starting Materials:
- Resorcinol (1,3-dihydroxybenzene) if not fully converted.
-
Isomeric Byproducts:
- The Kolbe-Schmitt reaction can produce positional isomers, such as 2,4-dihydroxybenzoic acid, if carboxylation occurs at a different position. The exact isomer distribution depends on reaction conditions.
-
Over-carboxylated Products:
- Trace amounts of dicarboxylic acids (e.g., 2,6-dihydroxyisophthalic acid) if excess CO₂ reacts with the ring.
-
Degradation Products:
- Phenolic compounds or quinones formed from oxidation or thermal decomposition of resorcinol or the product under reaction conditions.
-
Solvents or Salts:
- Residual solvents (e.g., water, ethanol) from recrystallization or inorganic salts (e.g., NaCl, KCl) from the acidification step, though these are typically minimized in a well-purified sample.
-
Trace Contaminants:
- Minor impurities from starting materials or equipment, such as metal ions or organic residues.
Quantification of Purity
The 98% purity claim is typically based on analytical techniques like HPLC (High-Performance Liquid Chromatography), NMR (Nuclear Magnetic Resonance), or titration. The certificate of analysis from the supplier might specify the major impurities if they’re significant. For most applications (e.g., research or industrial use), 98% is considered high purity, and the 2% impurities are usually negligible unless ultra-high purity is required (e.g., for pharmaceutical standards).
If you need a more specific synthesis route or impurity profile (e.g., for a particular supplier’s product), let me know, and I can refine the answer further!"
-
-
Interestingly 2,6DHBA is a fecal metabolite in humans strongly influenced by diet .
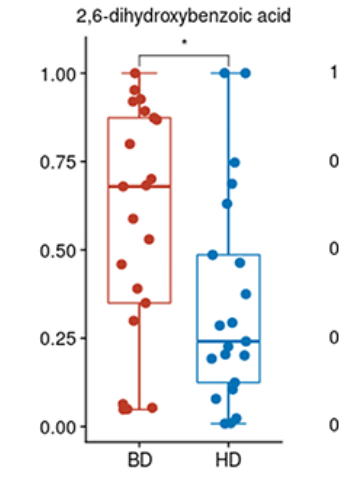
Apparently different benzoic acids are contained in fruits and other foods. And they alle seem to have anti-cancer properties . I suspect they undergo strong metabolism by the liver and the microbiome.
"Recent reports have demonstrated that the aspirin metabolites 2,3-dihydroxybenzoic acid (2,3-DHBA), 2,5-dihydroxybenzoic acid (2,5-DHBA) and the flavonoid metabolites 2,4,6-trihydroxybenzoic acid (2,4,6-THBA), 3,4-dihydroxybenzoic acid (3,4-DHBA) and 3,4,5-trihydroxybenzoic acid (3,4,5-THBA) were effective in inhibiting cancer cell growth in vitro. Limited in vivo studies also provide evidence that some of these hydroxybenzoic acids (HBAs) inhibit tumor growth in animal models."
" Interestingly, these HBAs are also abundantly present in fruits and vegetables. "
https://www.mdpi.com/1420-3049/25/9/2243