Casein with Thr and Cys or whey protein stimulate mucin production and protect against mild (DSS) colitis
-
"Whey protein contains bioactive proteins and peptides, such as lactoferrin and, in the case of whey protein obtained from the cheese-making process, glycomacropeptide that can be beneficial in preventing colitis (i.e., chronic inflammation of the large intestine; Togawa et al., 2002a,b; Daddaoua et al., 2005). Whey protein is also a rich source of threonine, cysteine, serine, and to a lesser extent proline (Walstra et al., 2005). These amino acids are major constituents of gastrointestinal secretory mucins (Tytgat et al., 1996a). Recently, it has been shown that supplementation of cysteine, threonine, proline, and serine increases mucin synthesis and improves histological changes in dextran sulfate sodium (DSS)-induced colitis in rats (Faure et al., 2006)."
"Cheese whey protein reduced DSS-induced diarrhea and tended to decrease DSS-induced fecal blood loss (
Figures 3 and 4), 2 major clinical signs of IBD, and diminished DSS-induced gene expression of inflammation markers. "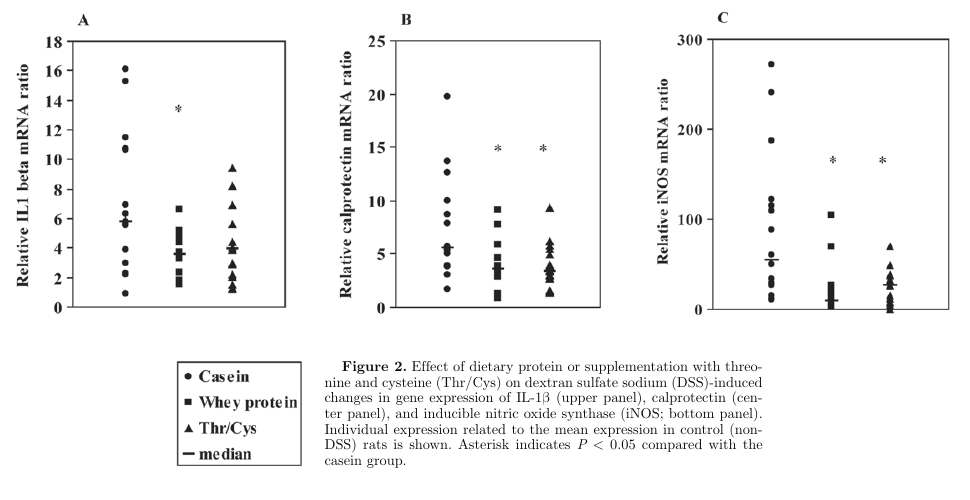
"Although the amino acids glutamine, glycine, and histidine have been shown to diminish clinical symptoms in animal models of IBD (Ameho et al., 1997; Tsune et al., 2003; Andou et al., 2009), it is unlikely that these amino acids explain the protective effect of cheese whey protein because the cheese whey diet was not enriched in these amino acids (Table 2). Because supplementation of Thr/Cys to the casein diet resulted in similar effects, it is most likely that the protective effect of cheese whey protein can be ascribed to its high Thr and Cys content."
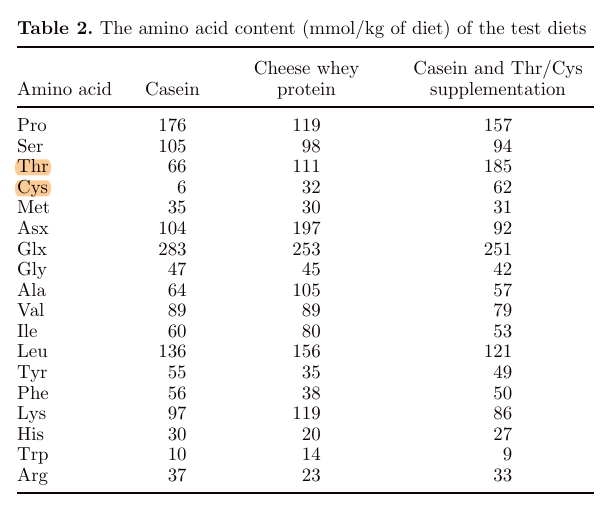
"Casein (4%) was added to the cheese whey protein diet to prevent limitation of the amino acids tyrosine, phenylalanine, and histidine."
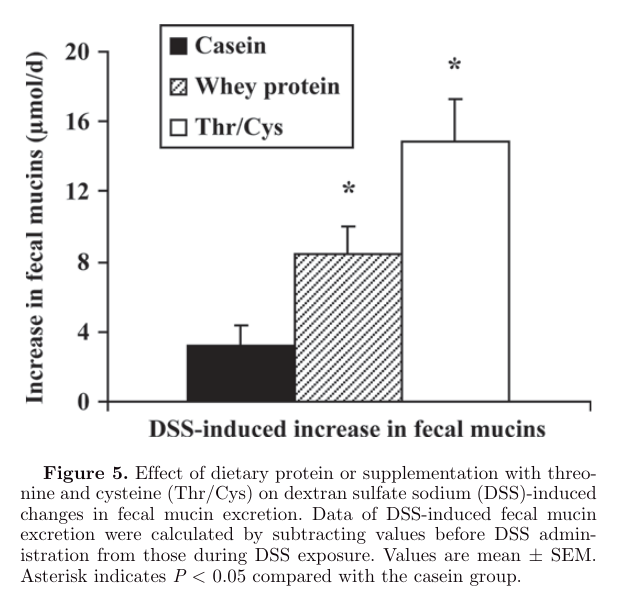
"In our study, fecal mucin excretion was already increased in rats fed cheese whey protein diets or casein diets supplemented with Thr/Cys before DSS administration. Because supplementation of threonine does not stimulate mucin synthesis in normal non-DSS-treated growing rats (Faure et al., 2005), a threonine-independent mechanism may account for the higher fecal mucin excretion under normal conditions. The whey protein α-lactalbumin, which is rich in cysteine, stimulates mucin production in gastric cells (Ushida et al., 2007), and the cysteine analog N-acetylcysteine has been shown to increase mucin expression in human airway mucosa ex vivo (Hauber et al., 2007). Thus, it is very likely that the cysteine moiety of cheese whey protein is responsible for the observed increase in fecal mucins under nonstress conditions. This finding may require further investigation."
"One of the mechanisms by which cheese whey protein diminished DSS-induced symptoms is by increasing mucin synthesis, as has been shown before for threonine, cysteine, proline, and serine supplementation (Faure et al., 2006). Because MUC2, the major secretory mucin of the colon, is decreased in colitis in humans (Tytgat et al., 1996a) as well as in animal models (Tytgat et al., 1996b) and plays an important role in preventing gut inflammation in mice (van der Sluis et al., 2007), enhancement of MUC2 synthesis by providing whey protein may protect against colitis. Although we did not measure mucin synthesis, our data points in this direction because fecal mucin excretion was increased despite unaltered colonic MUC mRNA levels in rats fed cheese whey protein (Figure 5)."
"Another mechanism by which cheese whey protein protects against DSS colitis can be by stimulating protective microbiota species. The intestinal microbiota plays an important role in the pathogenesis of IBD, because IBD patients show less biodiversity in fecal microbiota species and higher numbers of firmly adhered bacteria to intestinal mucosa compared with nondiseased subjects (Chichlowski and Hale, 2008), and oral administration of probiotic lactobacilli and bifidobacteria has been shown to prevent DSS-induced colitis in rats (Osman et al., 2004). In our study, cheese whey protein increased fecal counts of lactobacilli and bifidobacteria. Because secreted mucins may be used by the resident microflora as a prebiotic substrate (Schaafsma, 2007), the higher mucin levels in rats fed whey protein may account for the observed increase in fecal lactobacilli and bifidobacteria. Nevertheless, in our study, fecal lactobacilli and bifidobacteria counts did not correlate with fecal mucin contents (R = 0.32 and 0.24 for bifidobacteria and lactobacilli, respectively) indicating that whey protein enhances these microbiota species by another mechanism. The cheese whey protein constituents α-lactalbumin and glycomacropeptide may act as growth promoters for bifidobacteria (Petschow and Talbott, 1991). However, because supplementation of threonine and cysteine exerted the same effect on lactobacilli and bifidobacteria, cheese whey protein stimulates these microbiota species by its amino acid composition in a yet-unknown way rather than by specific individual whey proteins."
-
It sounds to me like you're saying we should drink milk instead of eating cheese.
-
Perhaps buffalo cheese could be even better
-
For the therapeutic purpose in question, milk must be better than cheese, but more whey protein deserves to be tried.
Inflammation markers:
- Whey protein < Casein + AAs < Casein
Colitis markers:
- Casein + AAs < Whey protein < Casein
Apparent mucin synthesis:
- Casein + AAs > Whey protein > Casein
Microbiota:
- Casein AAs ≈ Whey protein > Casein
Protein source Thr Cys Casein 1× 1× Milk (cow) 1× 2× Whey protein 2× 5× Casein + AAs 3× 10× Milk (from cows, as the most common) would flip the ratio of their whey protein diet.
Experiment (whey protein group):
- 20% Casein
- 80% Whey protein
Milk:
- 80% Casein
- 20% Whey proteins
People can experiment varying their proportion as they do for infants.
- How to adjust α-lactalbumin and β-casein ratio in milk protein formula to give a similar digestion pattern to human milk?
- Re-evaluation of the whey protein/casein ratio of human milk
- Effect of Modified Casein to Whey Protein Ratio on Dispersion Stability, Protein Quality and Body Composition in Rats
- The Balance Between Caseins and Whey Proteins in Cow's Milk Determines its Allergenicity
-
"As previously reported, acute inflammation severely affects colon metabolism by stimulating protein anabolism [4, 9, 18]. Because colon protein content was not modified along the colitis course and resolution despite a higher protein synthesis rate, this increase in biosynthesis might reflect colon repair after epithelium erosion, with possibly a higher epithelial cell loss in the lumen. The locally induced inflammation is known to provoke an increased amino acid need for protein synthesis in the colon, not only when inflammation is maximal (day 7–10) [19], but also likely during the healing process. This increase, notably at a time when dietary daily intake was reduced, could have been compensated by amino acids supplied by other tissues, such as muscle. A reduced protein synthesis in muscle would also spare amino acids for increased protein synthesis in the colon, liver and spleen. Indeed, muscle is a major contributor to restore protein and energy losses in situations where protein catabolism is manifest such as starvation [20], acute injury [21] or chronic inflammation [9]."
"The present study shows that the protein metabolism modifications are modulated by the dietary protein content. The moderately high-protein diet (P30%), which has been recently shown to improve epithelial repair after an acute colitis episode [14], was associated with a lower protein synthesis rate, restoring initial level in colon compared to the two other diets [14% and 55% protein]. It is noteworthy that several colitis induced alterations were restored earlier with P30 diet consumption compared to the two other diets, such as body weight, caecal protein content, spleen and muscle protein synthesis rates. These changes coincided with the recovery of P30-fed mice that showed less inflammation and bacterial translocation (as evidenced by measuring the circulating LPS-BP concentration [14]) and faster colon repair in accordance with an earlier restoration of initial protein synthesis colon as shown in the present study."
"Our finding that the P30 diet restores rapidly and sustainably the initial (before colitis induction) colon protein synthesis can be related to our previous study in which we showed that P30 diet improved (when compared to the P14 diet) the colonic mucosal healing process by accelerating inflammation resolution, increasing epithelial repair, reducing permeability, and inducing epithelial hyperproliferation [14]. This suggests that the P30 diet, by furnishing an increased amount of amino acids, helps in the restoration of the colon epithelium, thus explaining the less active protein synthesis at this site. However, the relative amount of amino acids that is devoted to the restoration of the colonic epithelium remains to be determined when compared to other amino acid-consuming processes involved in inflammation. We previously showed that a specific mixture of amino acids given to rats after colitis induction was able to improve the colon regeneration/re-epithelialization, and this amelioration was found to coincide with a reduction of the stimulated protein synthesis rate in the colon when compared to control animals not receiving the amino acid mixture [23]. In sharp contrast, by further increasing the protein content in the diet (P53), we did not observe in this study any effect on the colon protein synthesis rate when compared to the P14 diet. This result can be ascribed to the deleterious effects of the P53 diet on the disease activity index, both in intensity and duration after a DSS challenge [14]. The reasons that would explain how a very high-protein diet consumption, although furnishing more amino acids is counterproductive after colitis induction, are not clear. One possibility is that by increasing the protein content in the diet, a larger amount of the dietary and endogenous protein escapes digestion in the small intestine, and thus reaches the large intestine. As a consequence, the microbiota produces an increased amount of amino acid-derived metabolites, some of which (hydrogen sulphide, ammonium, p-cresol) being known to be deleterious for the colonic epithelial cells, notably in terms of energy metabolism [24]."
"In conclusion, our study highlights the severe impact of acute colonic inflammation not only on the metabolism of proteins in the splanchnic area, but also in the peripheral tissues. The ability of the P30 diet to restore a normal protein synthesis in the colon may correspond to its documented beneficial effect on mucosal healing. In addition, the recovery of the initial protein synthesis rate in the various tissues in the period of time following colitis induction appears to be modulated by the amount of protein in the diet, with the P30 diet being more effective than the P53 diet."