DNP - Soon to get Approved for Fat Loss in a Country Near You
-
Well, the study I linked showed no serious adverse events and certainly no deaths after 61 days at 450 mg per day of HU6.
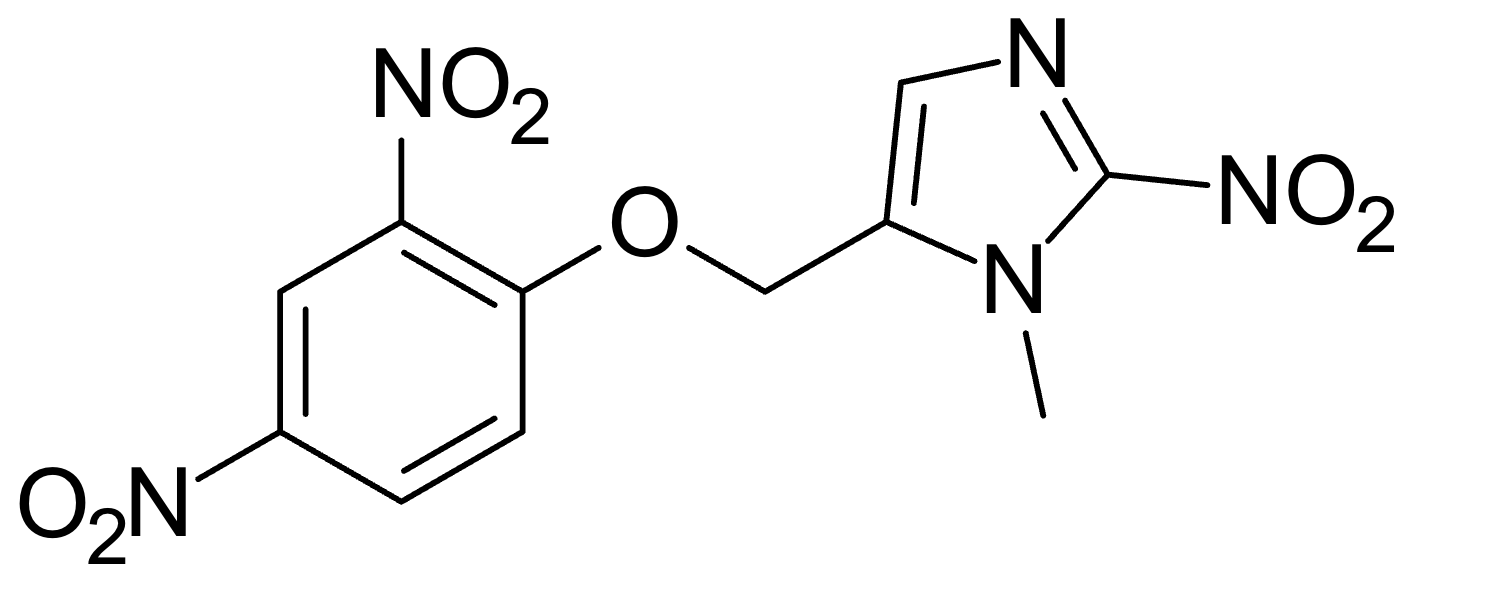
If you look at the molecular structure of HU6, it's clear that for every one molecule of HU6, there is one molecule of DNP.
The Dinitrobenzene moiety on the lower left obviously can convert to DNP when the C-O bond is broken
I assume for the rest of my argument that the 5 carbon ring on the upper right is not pharmacologically active, though that clearly need not be true. If it is pharmacologically active, then the equivalent dose would be even higher than what I estimate below.
We can try to calculate the molecular weight of the moiety attached to the core DinitroBenzene molecule to calculate an equivalent dose of DNP.
Molecular weight of DiNitroBenzene core of HU6 = 6(12) + 2(14) + 4(16) + 16 = 180d
Molecular weight of moiety attached = 12 + 3(12) + 2(14) + 14 + 2(16) + 1 = 123d
=> Overall weight = 303d => fraction of weight that is converted to DNP (assuming perfect enzymatic conversion) = 0.6
=> Assuming 80% conversion = 0.47
So, 0.6 to 0.47 of the 450 mg dose is converted to active DNP, given the 80% efficiency assumption (which is arbitrary) = 450(0.47) - 450(0.6) = 213mg - 267mg DNP equivalent from the highest tested dose = 450 mg.
-
@CrumblingCookie said in DNP - Soon to get Approved for Fat Loss in a Country Near You:
@alfredoolivas said in DNP - Soon to get Approved for Fat Loss in a Country Near You:
It definetley does accumalate though, which is why deaths are never instant - at least to my knowledge - so it must have quite a long half life.
Yes, we must not be deceived by short plasma half-lifes when in fact it accumulates within cells and tissues. Iirc of DNP fatalities they also showed increasing skin and tissue discoloration (yellow, as the DNP powder) and could die several days after their last dose. Giving a prodrug should simply extend that to even greater lengths?
It's reall not at all controllable by acute countermeasures. Wouldn't take it.If it does indeed accumulate, then wouldn't you see long lasting effects due to that accumulation? Or to put it another way, doesn't it mean you could just take one dose and be done until it clears your system?
-
@alfredoolivas The question is whether those metabolites:
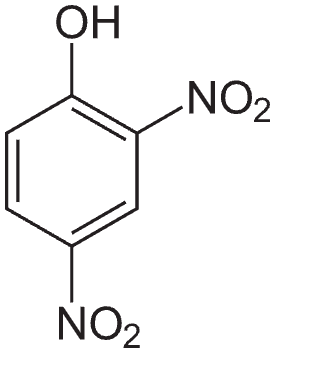
2-amino-4-nitrophenol and 4-amino-2-nitrophenol
are uncouplers or not.
At least the 4-amino variant appears not to be an uncoupler, nor does injecting it into rodents seem to cause cancer (see pages 6-9 below)
https://oehha.ca.gov/sites/default/files/media/downloads/proposition-65/chemicals/4am2ntro.pdf
The 2-amino variant appeared to decrease BW slightly only in male rats, was modestly toxic at very high doses to the kidneys, but is probably not carcinogenic. So, I doubt it is an uncoupler.
Therefore, it seems that those two metabolites are not uncouplers. If they were, we would have seen evidence of uncoupling in the rat models.
So, their long half-life is irrelevant as to the active life of DNP.


-
@engineer I don't think any uncoupling agent does accumulate. Certainly the 2 and 4 amino metabolites I mentioned are not uncouplers.
The yellow color could be because of those metabolites, which themselves are yellow but are not uncouplers.
-
@jamezb46 Phenol it's self has a 4.5 hour half life. When you add two nitrogroups, it will increase its half life. DNP is yellow itself.
-
@alfredoolivas Right. So this is where we are right now I think as far as the "accumulating" theory of DNP.
The rodent models I posted suggested a short (hours) half life, and the human data you supplied from the FDA suggest a 10.3 hour half life.
The 10.3 hour half life seems consistent with my findings that fatalities usually occur around 7 hours after an overdose.
What does seem to accumulate are the 2-amino and 4-amino nitrophenols. These would explain lasting yellow pigmentation of various tissues. But those are not uncouplers, though they are probably toxic.
-
kind of a side topic but isnt dnp an explosive? is that also why it is an uncoupler? the fact it has all this energy in it?
-
@jamezb46 The FDA half life is "estimated".
If you look at the blood concentrations of lucky survivors and look at the effects & timescale of events, it's pretty obvious it accumalates. At least in humanskind of a side topic but isnt dnp an explosive? is that also why it is an uncoupler? the fact it has all this energy in it?
Phenols are uncouplers. Salyilic acid has a phenol group and is one. It does so non genomically I don't understand the mechanism.
-
@sunsunsun I don't know if this comment is trolling but it's extremely stupid.
The reason DNP is an uncoupler is because it is a protonophore, meaning it can carry "protons" aka hydrogen ions across the mitochondrial membrane thus causing proton "leak" and thus less energy efficiency.
There are also physiological uncoupling mechanisms that have to do with uncoupling proteins that are stimulated by thyroid hormones and some other hormones.
-
@jamezb46 xD