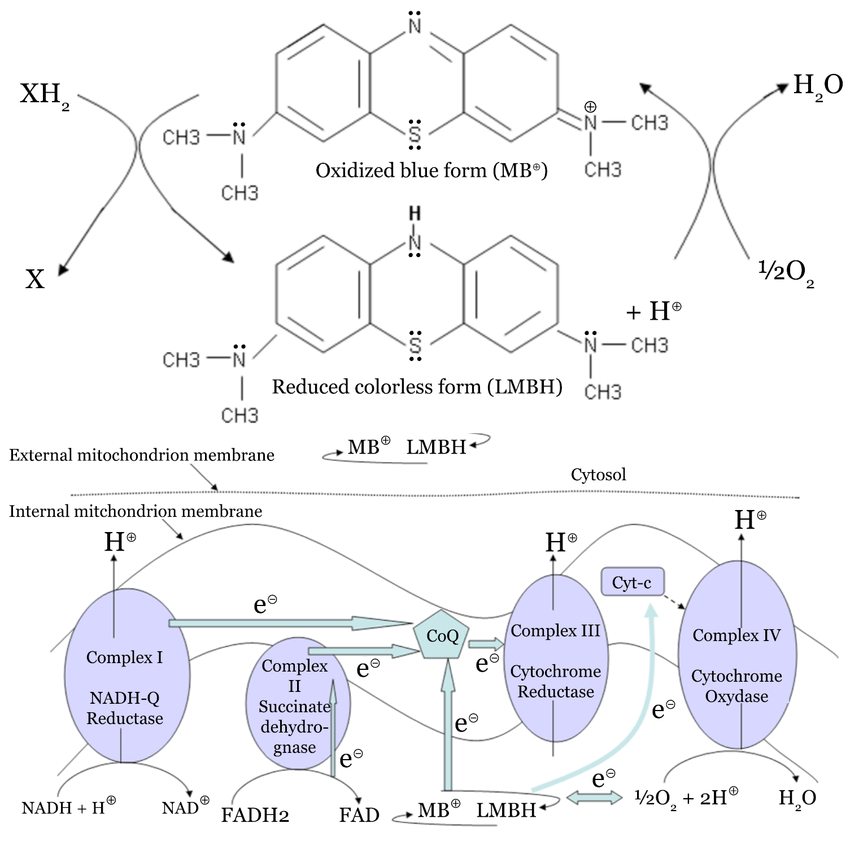
Different route of methylene blue in mitochondria (3rd way to get some atp & co2, bypass mitochondria complexes)
-
Heard ray say something interesting in a podcast, about how methylene blue can be used to stimulate respiration get co2 & atp from mitochondria even when complexes I and III are not functioning properly, bypassing them and acting as a bridge to get electrons to complex IV. & helps establish more complex IV through cytochrome c
these are the studies
https://www.mdpi.com/2076-3921/10/2/305
https://pubmed.ncbi.nlm.nih.gov/25277417/theres another very interesting thing about it , it stimulates ATP/co2 production even with an absent OXPHOR system / transport chain.
Only glycolysis = 2 ATP gained per glucose
Glycolysis + tca cycle + mitochondrial respiration =up to 38 ATP gain & co2up to ~32 atp gain, typically a bit less with typical mild efficiency loss, + gain co2
Glycolysis + tca cycle (methylene blue enabled) = 4 ATP gain and co2 gain, with an increase in metabolism creating moreSo in states of defective mitochondria transport chain (poisoning, dietary or environmental toxins, birth defects, deficiencies, etc) or even lacking, methylene blue can provide extra ATP & co2 that the cell needs
also does it in a way that lowers pyruvate going to lactate, lowers lactate , and still through acetyl COA being oxidized to produce co2
"METHYLENE BLUE INDUCED O2 CONSUMPTION IS NOT DEPENDENT ON MITOCHONDRIAL OXIDATIVE PHOSPHORYLATION: "
We present evidence that after being reduced into leuco-methylene blue (LMB) in presence of reducing molecules that are physiologically found in cells (such as NADH), the re-oxidation of LMB by oxygen can account for the increased oxygen consumption observed in vivo.
In conditions of acute mitochondrial dysfunction, these MB redox cycling properties allow the rescue of the glycolysis activity and Krebs cycle through an alternate route of oxidation of NADH (or other potential reduced molecules), which accumulation would have otherwise exerted negative feedback on these metabolic pathways.
Our most intriguing finding is that re-oxidization of MB by oxygen ultimately results in an in vivo matching between the increase in the rate of O2 consumed, by MB re-oxidation, and the rate of CO2, produced by the intermediate metabolism, imitating the fundamental coupling between the glycolysis/Krebs cycle and the mitochondrial respirationWe first show that intra-venous administration of micromolar levels of methylene blue in sedated and mechanically ventilated rats, increases not only resting oxygen consumption but also CO2 production (by ~ 50%), with no change in their ratio.
Notably, the large increase in cellular oxygen consumption caused by MB was relatively indifferent to the status of the mitochondrial respiratory chain: oxygen consumption persisted even when the respiratory chain was inhibited or absent (using inhibitors and cells deficient in mitochondrial oxidative phosphorylation); yet MB did not impede mitochondrial ATP production in control conditions.
A simplified description of metabolism would state that, on the one hand, metabolism oxidizes carbon-containing substrates, reduces coenzymes (NAD, FAD/FMN) and releases CO2 (glycolytic activity and Krebs (TCS) cycle); while on the other hand, mitochondrial respiration (Ox in figure 11) oxidizes these coenzymes and consumes oxygen. The relationship between Metab. and Ox is closely coupled through of positive feedback system, wherein accumulation of reduced coenzymes (NAD, FAD/FMN), due to a depression in OX for instance, will decrease Metab. Accordingly. Conversely, any increase in Ox entrains a similar increase in Metab. The overall purpose of this is to convert energy liberated by oxidation into the regeneration of the ATP continuously used by the cell for sustaining its energy need
MB also possesses antidotal properties in conditions of acute poisoning of mitochondrial respiration. We will use hereafter a simplification of this respiration that oxidizes of substrates into carbon dioxide and consumes of oxygen as the sum of two processes: “metabolism” that oxidizes substrates into carbon dioxide and reducescoenzymes (NAD or FAD) and “oxidative phosphorylation” (OxPhos) that re-oxidizes the reduced coenzymes (NADH, FADH2) and reduces oxygen into water. The energy liberated by >these oxidation reactions is converted into ATP to feed cellular energy needs. The reduction/oxidation cycle of MB may be considered either as a modifier of or as an alternative to OxPhos
{maybe can in part of its effect carry electrons to bypass complex III to get to IV?, but}
While this mechanism appears attractive, it cannot explain the beneficial effects of MB when complex IV is inhibited by cyanide or hydrogen sulfide that prevent oxygen reduction by complex IV.
Yet, it has been consistently reported MB increases O2 consumption in
cell culture (Barron, 1930; Bodine and Lu, 1950; Harrop and Barron, 1928), regardless of the complex that is blocked, suggesting that such an increase in VO2 during MB exposure, is not the result of a restoration or an increased activity of the mitochondrial electron chain per se, but could rather reflects the rate at which LMB is directly re-oxidized by O2 into MBIncreasing concentrations of MB (0, 2, 4, 8 10, 25, 50, and 100
µM) induced similar VO2 increase in both cell types. {lacking oxphor vs normal}For ATP production from both glycolysis and mitochondrial respiration, addition of 50 µM MB into the reaction medium tended to decrease ATP production in the absence of the specific inhibitors
^ high amounts of methylene blue = less ATP yield when complexes weren't inhibited
In contrast, but only for ATP synthesis by mitochondrial respiration, it increased the rate of ATP synthesis in the presence of the specific inhibitors of the involved ATP synthesis pathway.
direct re-oxidation of NADH by MB could take place in the absence of enzyme
(figure 10). Therefore, according to the simplified view presented above, MB could divert metabolism and/or NADH re-oxidation, rather than to feed the Ox-PhosThe cytosolic glycolysis is a significant contributor to MB reduction (Figure 8), an observation that reproduces initial observations on MB influence on metabolism (Barron, 1930). This has the consequence to divert the reduction of pyruvate into lactate toward reduction of MB
Consistently, in rat model, the blood levels of lactate and pyruvate showed profound decrease in the lactate/pyruvate ratioThe reduction of MB by glycolytic enzymes would not in any way harm the
bioenergetic side of glycolysis (substrate linked ATP formation). Moreover, it could improve pyruvate availability for mitochondrial oxidation. In our experiments, the increase in VO2 brought by MB appeared almost unchanged under conditions of mitochondrial poisoning (Figures 3, 4) or with cells deficient for respiratory complexes
as if MB reduction/re-oxidation would be indifferent to the mitochondrial status.With respect to energy expenditure, VO2, VCO2 and reductive stress, the
consequences of the MB-induced “metabolic diversion” are identical to those induced by uncouplers. However, an important difference is that any negative impact on Ox would decrease the cellular tolerance to uncouplers but not to MB, which at the opposite rather improves outcome when Ox is compromised. {i guess as when the oxygen level drops with MB it stops converting, so is self regulating with this}Molecular oxygen is the substrate allowing re-oxidation of LMB. Of importance, MB
did not induce any change in the rat respiratory quotient, implying that the final product of the reaction was H2O and not H2O2. Two different mechanisms may explain how H2O is the final product: i) H2O2 is not produced and re-oxidation of LMB yields H2O. ii) Conversion of H2O2 into H2O and h2O2
.... Re-oxidation of LMB by O2 into H2O2 may therefore extensively contribute to the
metabolic effects of MB and fast conversion of H2O2 into O2 and H2O would result in a same final stoichiometry than that obtained with normal cellular respiration (supplementary figure Y). In addition, H2O2 release is not to be a serious concern, as long as catalase or equivalent antioxidant defenses are operativeAnaerobic glycolysis ensures the production of two ATP per glucose. In presence of the MB/LMB redox cycle, metabolism and substrate linked ATP formation (4 ATP/glucose) could continue in absence of Ox. . Second, MB accelerates the metabolic rate up to three times. For instance, assuming that, the maximal effects of MB could lead, by a full oxidation of glucose and Acetyl CoA, to 12 ATP ad 3 ATP respectively, the ATP production rate caused by a maximal MB effect could account for ~ 40% of the total ATP production if the yield of Oxphos (coupling) that would be ~ 80% of normal Oxphos (assuming that 100% Oxphos activity will lead to 30.2 ATP and 8.1 ATP through glucose and acetyl CoA full oxidation respectively), or even ~ 60% for a yield of Oxphos at ~ 50% of normal. These figures could certainly explain the beneficial effects reported for MB in various pathological conditions, associated to mitochondrial bioenergetics impairment, such as cyanide or hydrogen sulfide intoxication
{^ matches a mice study i saw where ~5mg heq partially restored mitochondria atp in copper depletion model){in normal rats, a big dose of methylene blue increases metabolic rate / CO2 / temp in a big burst , but its short lived as only elevated a little above baseline by 60 minutes - the main use of this is where there's significantly dysfunctional mitochondria to make up some of the lacking atp & co2 through an alternate approach. also this higher atp & co2 might then help re-structure the mitochondria to regain fuller production.
-
@cs3000, your findings are great.
Their writing is a bit confusing at times, possibly a result of English not being the main language.
"In presence of the MB/LMB redox cycle metabolism and substrate linked ATP formation (4 ATP/glucose) could continue in absence of Ox. Two ATP come from oxidation of acetyl-CoA in the Krebs cycle (1 ATP/Acetyl-CoA). [..] Two factors would make MB more efficient than predicted from a direct simplistic biochemical calculation: first, in contrast with substrate linked phosphorylation, the production of ATP by Oxphos is not stoichiometric and takes place with a yield directly dependent on ionic leakage across the mitochondrial inner membrane (Mitchell chemiosmotic theory), while the theoretical values above consider a yield of 100%, 80% appears a reasonable assumption but depending on tissues values as low as 50% are considered (Jastroch et al., 2010). Second, MB accelerates the metabolic rate up to three times. For instance, assuming that, the maximal effects of MB could lead, by a full oxidation of glucose and Acetyl CoA, to 12 ATP ad 3 ATP respectively, the ATP production rate caused by a maximal MB effect could account for ~40% of the total ATP production if the yield of Oxphos (coupling) that would be ~80% of normal Oxphos (assuming that 100% Oxphos activity will lead to 30.2 ATP and 8.1 ATP through glucose and acetyl CoA full oxidation respectively), or even ~60% for a yield of Oxphos at ~50% of normal."
Complete glucose metabolism:
Substrate-level phosphorylation
- 2 ATP/glycolysis
- 2 ATP/2 TCA cycles
Oxidative phosphorylation
- 25 ATP/10 NADH
- 3 ATP/2 FADH2
Totaling about:
- 32 ATP/glucose (100%)
- 26 ATP/glucose (80%)
Oxidation of NADH and FADH2 without respiratory complexes through MB would maintain the substrate-level phosphorylation contribution:
- 4 ATP/glucose
The oxidation of acetyls is already quantified, there is no 12 ATP
+ 3 ATP[from 3×(4 ATP+ 1 ATP)]. Acceleration can also be accomplished by relying on glycolysis alone to produce ATP, but both methods are quite wasteful (4 or 2 ATP versus 26 ATP per glucose). The main advantages of this alternative appear to be in allowing (pyruvate dehydrogenase and) the TCA cycle to keep functioning to metabolize glucose completely and not depending on lactate accumulation to regenerate NAD+. -
@Amazoniac
ah yeah i misread that thanks. so with MB vs glycolysis alone, you get an extra 2 atp from acetyl-coa (and co2) and the 2 atp (gain) from the first step
it stops there, so double the ATP+ a co2 gain vs if there was only glycolysis through enabling the tca cycle by different means, and ramping up of metabolic rate gives the 12 atp -
They published it that way, might be a result of:
Those 4 ATP from 'substrate-level' phosphorylation won't change, and neither should the reduced coenzymes formed throughout:
- 10 NADH/glucose (to be oxidized in Complex I)
- 2 FADH2/glucose (to be oxidized in Complex II)
⠀ - 12 'reducing equivalents' in total
But the respiration component may yield more or less ATP depending on the complexes skipped. As discussed elsewhere, the synthesis of 1 ATP consumes approximately 4 H⁺:
- 0.25 ATP/1 H⁺
Therefore:
- Complex I: 4 H⁺ → 1 ATP × 10 → 10 ATP
Complex II: none cross- CoQ
- Complex III: 4 H⁺ → 1 ATP × 12 → 12 ATP
- Cyt c
- Complex IV: 2 H⁺ → 0.5 ATP × 12 → 6 ATP
In ascending order (from complete to no skipping):
CI|CII ↷ CoQ ↷ CIII ↷ cyt c ↷ CIV: ⠀4 ATP (4 ATP + 0 ATP)CI|CII ↷ CoQ ↷ CIII ↷cyt c ↷ CIV: 10 ATP (4 ATP + 6 ATP)CI|CII ↷CoQ ↷ CIII ↷ cyt c ↷ CIV: 22 ATP (4 ATP + 6 ATP + 12 ATP)- CI|CII ↷ CoQ ↷ CIII ↷ cyt c ↷ CIV: 32 ATP (4 ATP + 6 ATP + 12 ATP + 10 ATP)
-