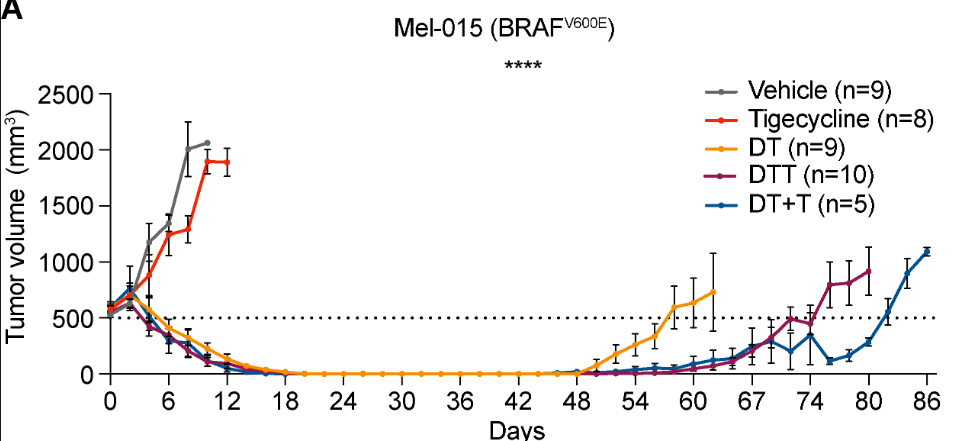
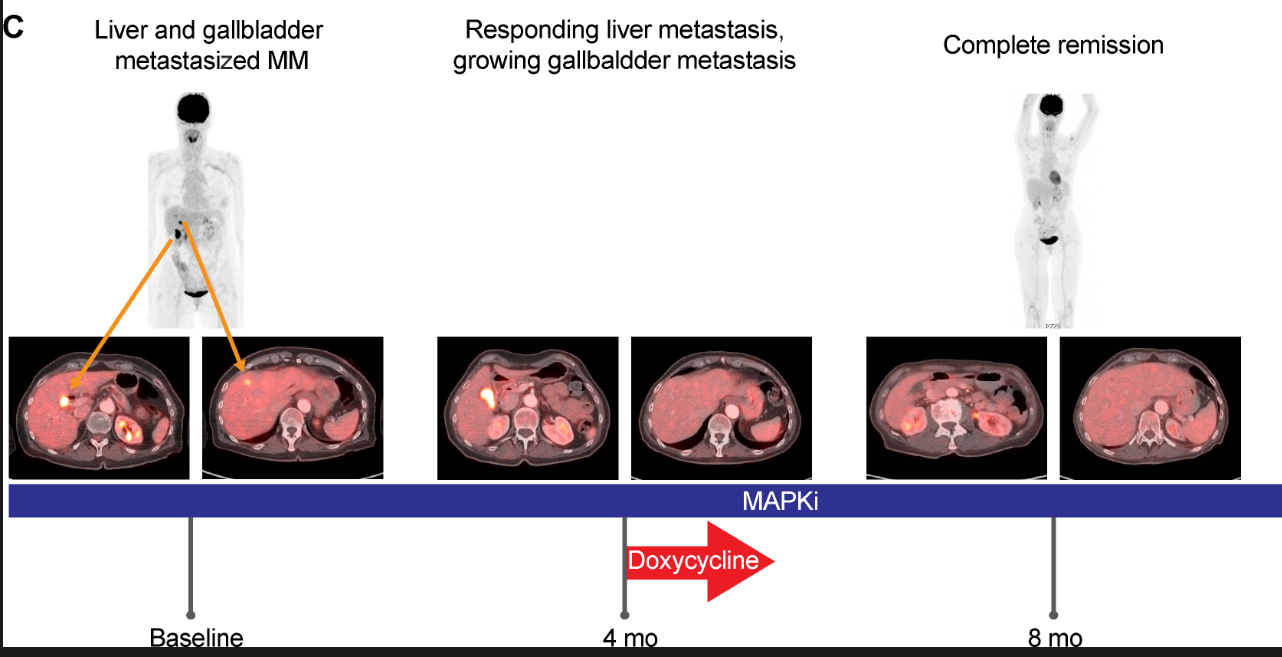
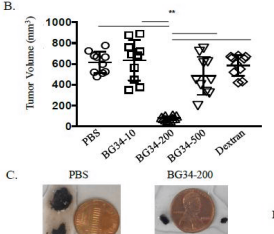
interesting find - if u target a main 2% subset of melanoma cells the tumor goes in most cases without destroying the other part. if some are left theres potential for the regression to return
(does doxycyline help these ones get destroyed when combined with inhibitors?)
https://www.pnas.org/doi/10.1073/pnas.1009069108
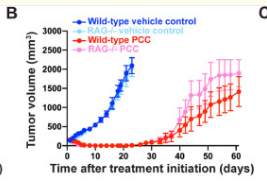
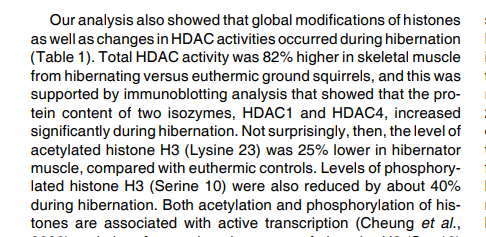
we revealed that established tumor lesions can be efficiently eradicated by targeted elimination of the less than 2% subset of melanoma cells with the CD20+ high molecular weight melanoma-associated antigen (HMW-MAA)+ phenotype without targeting the tumor cell mass, whereas targeted elimination of any minor subset is less effective.
These data provide a strategy for improving the biological therapy of melanomas and, moreover, for redesigning current drug development paradigms used in the treatment of cancer.
HMW-MAA+ cells gave rise to melanomas upon transplantation, whereas sorted HMW-MAA− cells from the same melanoma did not.
indicating that these cells exhibit melanoma-initiating capacities.
T cells with redirected specificity for CD20 eradicated established melanoma lesions in four out of five melanoma biopsies, although the targeted cells represented less than 2% of melanoma cells.
It is noteworthy that transplanted tumors of patient 5, in which no CD20+ melanoma cells were detected, could not be eradicated by a CD20-redirected T-cell attack.
Where tumors were eradicated, no tumor relapse occurred during the observation period of >36 wk.
Because no tumor relapse was observed in any case during the >36 wk observation period, such melanoma eradication is obviously long lasting
We conclude that CD20 is not functionally crucial but marks a specific cell population. The unexpected observation, however, is that targeting the minor CD20+ melanoma cell population is as effective as targeting the majority of tumor cells. A caveat for a broad therapeutic application is that obviously some melanomas, in our cohort one out of five patients, do not harbor CD20+ melanoma cells
The observation that the specific elimination of a minor subset of tumor cells results in eradication of established melanoma lesions is crucial for the understanding of the biology of melanoma. Continuous melanoma growth obviously requires the presence of a minor population of cells, which displays the CD20+ HMW-MAA+ phenotype;Elimination of these cells is obviously required to eradicate the melanoma irrespective of the bulk of tumor cells