William F Koch Official Research Page
-
Hi friends. Just found this forum today and thought I’d share this Koch website as a hello, how you doing, meet and greet.
Happy reading y'all!https://williamfkoch.com/dr-kochs-publications/
William F. KochThe sole purpose of this site is to accurately present Dr. Koch’s theories to the scientific community in order to improve the well being of mankind.
This site chronicles over fifty years of Dr. Koch’s scientific research as he investigated and eventually isolated the most fundamental factor common to all disease. Dr. Koch’s epoch making discovery of this Least Common Denominator led to the ‘birth of a new science.’
Through extensive documentation Dr. Koch outlined the chemical processes by which disease may be reversed. Dr. Koch’s research focused on the means to restore the body’s oxidation mechanism back to its original vitality, thereby re-equipping the body with its innate ability to restore and maintain health, not only in cancers but also in a host of its ‘allied diseases.’
This research led to Dr. Koch’s development of several synthetic antitoxins: Glyoxylide, Malonide and PBQ. These catalysts became the stimulant necessary to achieve the oxidative separation of the ‘host cell/pathogen integration,’ when the pathogen was a virus, a carcinogen, a bacterial toxin or an incompletely burned tissue metabolite. Dr. Koch successfully defined the position of the activated amine group, the free radical, the double bond and the Carbonyl group in pathogenesis and in its correction.
Of historic significance is the knowledge that as early as 1919, Dr. Koch’s discoveries were taking him in a direction diametrically opposed to the position held by Organized Medicine, which at that time was investing heavily in the development of radium and surgery as the most promising treatments for cancer.
After failing in its attempt to gain sole control over his research, Organized Medicine launched a fifty-year, unlimited assault aimed at discrediting Dr. Koch’s reputation, medical practice and research, along with those of any physician who dared to validate his Theories or use his Reagents. Organized Medicine developed an extensive propaganda campaign, disseminated false information on Reagent chemistry and publicly dismissed the Koch Theories, which emphasized the relationship between environmental toxins, dietary deficiencies and a depleted oxidation mechanism, as primary initiators of the disease process.
Because Dr. Koch endured such extensive persecution in regard to his science, he determined that the medical/pharmacological industry would forever remain unwilling to independently monitor, document or validate any of his ongoing laboratory research or medical case histories; therefore since his death, December 9, 1967, there have been no authentic Koch Reagents reproduced. It was because of the scurrilous intentions held by the medical/pharmacological industry that Dr. Koch intentionally withheld specific knowledge required in the production of viable Koch Reagents. (Therefore, any claims to the contrary should be viewed as suspect.
-
@dapose interesting stuff so his take on immunity , instead of trying to destroy the pathogens, giving them the conditions needed for proper functioning so they dont go into a hyper proliferative mode through dysfunction (like cells & cancered cells).
William Frederick Koch succeeded to break the toxic free radicals with partly cyclical, partly aliphatic structured carbonyl high oxido-redox potential and thus to eliminate the pathogenic blockages, due to his profound biochemical knowledge and resources. The preparations, their indications and methods of use as well as the complementary protien restrictive diet are explained below.
continuation of koch's research by 1 of his associates & his son posted by a translator https://lowtoxinforum.com/threads/dr-william-frederich-koch-usa-brazil-drs-erich-dieter-reinstorff-germany-continued-research.10800/
It is also evident that the data shows that the pathogenicity was not removed by a destructive reagent, but rather by the corrective function of the activated Carbonyl group. There was no longer any necessity for parasitism to win survival and no forced effort at reproduction was necessary to try to overcome a deficiency, for the correction was already made by supplying the normal source of energy for its created function and hence, pathogenicity could not result
And this leads to the question posed by Warburg as to the irreversibility of cancer which we show exists only so long as the carcinogen is integrated with the cell’s energy producing and receiving mechanisms for function and mitosis, and which is reversed by oxidatively removing the integrated carcinogen, virus, etc.One concludes, that bacteria and tissue cells regained the energy utilization powers required for their normal metabolism and function and thus, their parasitism and pathogenicity was lost.
It is also to be noted that the Reagents were given in such high dilution that the amounts represented only 10-(12) grams per dose, which obviously is not an injurious dose, but instead could only be a catalytic constructive dose. The conclusion therefore follows that the bacteria and tissue cells are restored to their normal places in the biological economy by these two atomic groups and that disease germs, in active and suppressed focal infection, loose their pathogenicity whether they are arsenic fast, antibiotic resistant, or otherwise unresponsive to medications, like many trypanosomes,
Our Postulate is thus supported in this respect, so that when a germ’s dehydrogenating power is crippled, it cannot obtain energy for survival in the normal way by making harmless oxidation products, but instead produces such toxic products as toxic amines and hemolysins, which cause disease. It is not surprising, therefore, that after contact with the Reagents of this text, the dehydrogenating power would be restored and isagain able to oxidize to harmless materials, the toxic products it had formerly produced
Carbonyl, free radicals and molecular oxygen are the principles. Electron spin resonance techniques show that all aerobic tissues contain free radicals, so long as they are alive. (Schoffa 1964) Cancer cells are shown to contain less free radicals than normal tissues. This is of course in proportion to their anaplasia and inability to function oxidatively. Any increase in free radical content above that of normal tissue must be attributed to the polymerizing of the carcinogen in proportion to its malignancy, and as activated by magnetic influences, magnetic storms, etc.
These techniques show also that gamma rays are able to destroy Carbonyl groups and thus, tend to make the tissue metabolism of the malignant order, as in Warburg’s Oxygen Starvation Techniques and with complete reversibility, for the addition of quinones or other Carbonyl structures (mentioned in the text) restore the normal oxidative progression, when oxygen is also admitted. This is another confirmation of our Thesis that the Pasteur Effect is a function of the Carbonyl group, and unless the functional Carbonyl group is present to dehydrogenate, when oxygen is admitted, the Pasteur Effect will not be observed.Inactivation of bacteria, reduction of inflammation by gamma rays and negative consequences from the use of modern toxic amine antibiotics, may be considered also under this heading.
Copper is mainly diamagnetic, its oxide being weakly paramagnetic. Still its presence is essential to the action of polyphenol oxidase, vitamin C, the Ubiquinones and for the utilization of iron in hemoglobin and other activities. We found copper, as supplied by the waters of the Great Lakes, as essential to the best success of our Reagents in the treatment of cancer for nearly half a century. Where the tissues held a good quota, such successes as illustrated in, “The Integration of Pathogen and Host Cell Critical Atomic Groups and Their Separations" were the rule.compounds mentioned for the carbonyl group
None of the African or Australian pigments have been identified with medicinal activity. And still we may do so simply on the basis of the Carbonyl arrangements in each, as they all conform, including the Brazilian product, to the laws we have identified with anti-carcinogenic activity. The Brazilian pigment has not been identified as to structure except by ourselves, since we find it to be both the para and ortho forms of a naphthoquinone in isomerism, in two different trees. Here they can be extracted from the inner bark, and are named the Pau d’Arco Amarilo and Roxo, for the paraquinone and orthoquinone forms respectively. Both are proven to be curative in cancer and other diseases, the ortho form being most active in conformance to our Thesis. Both are of lower oxidation-reduction potentials than the Synthetic Agents we offer in the text, and run from 0.3 to 0.9 volts lower than our Reagents including: Glyoxal, Methyl Glyoxal, Rhodizonic acid, Triquinoyl, Compound C and the long straight chains of Carbonyl groups of the text, simple Parabenzoquinone and Diphenoquinone. They hence have a much-limited field of activity and are adapted to continued use over long periods by mouth, in which form they meet the needs of primitive people. However, as civilization has changed disease systems, the Synthetic Products we have used for the past fifty years, which were kept from the sufferer by bureaucratic and commercial interests, must be awaited by present and future generations. It will be of interest also that Rhodizonic acid, being stabilized and though reduced in activity by the two hydroxyl groups, is of use when taken by mouth and will thus serve those who cannot afford professional assistance. The structural patterns of the others can be represented by that of the Australian pigment Lamatol or the African Lapachol with but slight changes in their side-chains.
Conclusions:
Through blocking of the functional carbonyl group, for instance, the energy producing (ATP-) cellular metabolism, toxic byproducts can originate in metabolism from non-physiological free radicals, especially as a nitrogen bond that, for example, contain a very solid azomethine double bond ( R-C=N bond). Today we know that free radicals outside of cells are almost always toxic. Also, many forms of toxic free radicals can originate inside of cells due to, for example, ionizing radiation. The cell dividing mechanism can be so damaged that: a. cancer cells can originate uncontrollably, and b. the normally available destructive processes to these cancer cells are also reversibly damaged. If it is successful to reactivate the functional carbonyl group, or to remove the toxic bond (for example the azomethin double bond) through oxidation, or respectively dehydration, the malignant growth process can be reversed. It is imperative from this that out of all the possible free radicals that can be recognized by us as useful, only those having effects inside the cells and not outside the cells, must be activated.
Basic therapeutic ideas The immense importance of the pioneering work of Prof. W.F. Koch can be clearly seen in all of the current combinations. He recognized the radical intracellular metabolic processes necessary to sustain natural immunity and made them therapeutically useful. The application of ring and chain structured carbonyl groups are able to remove metabolic blocks under suitable conditions, that were decisively responsible for the cause and continuation of cancer growth. The suitable structure and concentration of these carbonyl group carrying molecules are drawn away from the Acetyl-CoA in their chain structure, and from their ring structure in the quinone-cascade of the cell's own respiratory chain.
The active orthomolecular carbonyl > C = 0 is the most important of all free radicals within the essential biochemical processes. The reversible double bond between carbon and oxygen is this radical protagonist of the electron dynamics. This maintains and regulates the whole metabolism, as well as all energy conversion processes and cellular functions
For Koch, the most widespread and dangerous disruptive factor of energy balance is overloading with nitrogen - rising from amino acids or alkylating agents, resulting in the displacement of the oxygen atom from the bond > C = 0 through N and the change to > C = N -C--. These non-physiological "azomethines" are highly toxic, and, unlike most free radicals stable and durable. They are deleterious in their purpose to the mentioned enzyme blockages and other dysregulation.
The azomethine groups can only be reconverted to stronger energy and higher redox potential> C = 0th through catalysts. This simultaneously manages the
regression and reintegration of malignant cells, highly toxic viruses and other
pathogens in the normal tissue metabolism. Koch availed himself of the derivatives he developed, that were compatible without secondary damage or side effects:
-
Page 58 & 59 (55 & 56 of archive) really interesting https://archive.org/details/TheChemistryOfNaturalImmunityWilliamKoch/page/n55/mode/2up
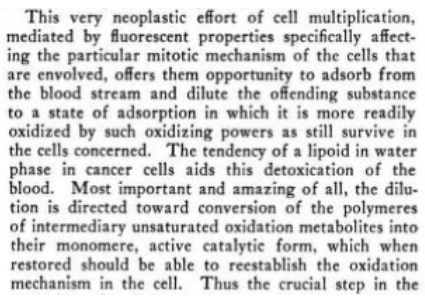
Kochs idea was the hyper-proliferation seen in cancer was an attempt at diluting energy disrupting substances so they can be more easily oxidized, from their distorted large polymer forms back to forms that can participate in cells functioning
"Some day when the multiplication is rapid enough compared with the production of the toxic factor the evolutionary purpose of the whole effort will be realized, and an efficient oxidizing machine will be produced which should serve as a protection not only against malignancy, but also against all of its associated allergic expressions, old age changes, and the lowered resistance against infection. "
-
@cs3000 & page 58 of archive
unsaturated carbon chains in some compounds , oxidizing (adding oxygen or another electron acceptor) = carbon dioxide produced , hydrating (adding water) = glucose produced
{interesting how that fits with cancer cells going by these reactions, co2 not produced as low oxygen to add, cancer cells water logged / more free water so producing more glucose for glycolysis}
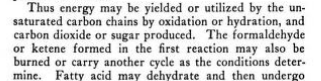
-
@cs3000
This stuff is all so fascinating to me! I had never seen that before in the old forum. I am a total novice lay person. I just like finding things that I heard Ray talk about and share with people like yourself who can run with these ideas! I personally love the quinones from Pau D’arco bark, cascara sagrada. I also love emodin from aloe Vera juice. Emodin is a natural hydroxyanthraquinone compound found in Aloe vera juice, specifically in the latex of the plant. Antibacterial, antifungal, and anticancer effects. But, yes! Thanks for diving into Kocks stuff and sharing! It’s helping me explore it more too!
Peace! -
@dapose thanks for the reminder, wanted to look into this guy since i heard peat mention his stuff on immunity without reliance on the immune system. seems like one of the grand masters . some stuff is going over my head but parts that stood out so far


- buildup of clunky large molecules (polymers) when a surplus of oxygen isnt supplied to the cell (or low amount of oxidation catalysts) , only a small amount of things that can join are taken out so more stuff combines and makes cellular process inefficient with these in the way:


-
hard to burn peroxides

-
malignancy of cancered cells and their loss of oxidizing power going together, and showing higher amounts of polymers , duplicating as attempt to dilute the molecule faster than its being added / created


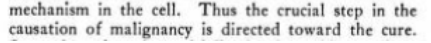

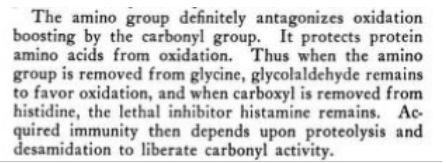
-
importance of carboxyl groups
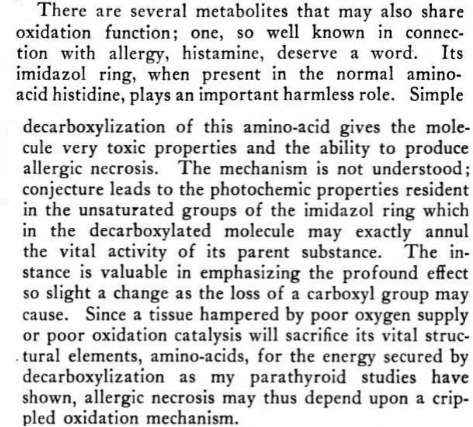
-
(interestingly he says all carcinogenic compounds have a fluorescent property)

- ? providing a competitive energy carrier with similar structure that doesn’t divert sugar / fatty acids away from being fully utilized and to cell division instead, supplying a normal oxidation carrier, that has 2 types of unsaturated groups which can competitively absorb heat and redirect energy to sugar & fat oxidation instead of hyperactivating other processes without being able to be carried far enough

- ,

-
This is the interview with Ray where he really goes into what he knows about William F Koch’s work.
Youtube Video