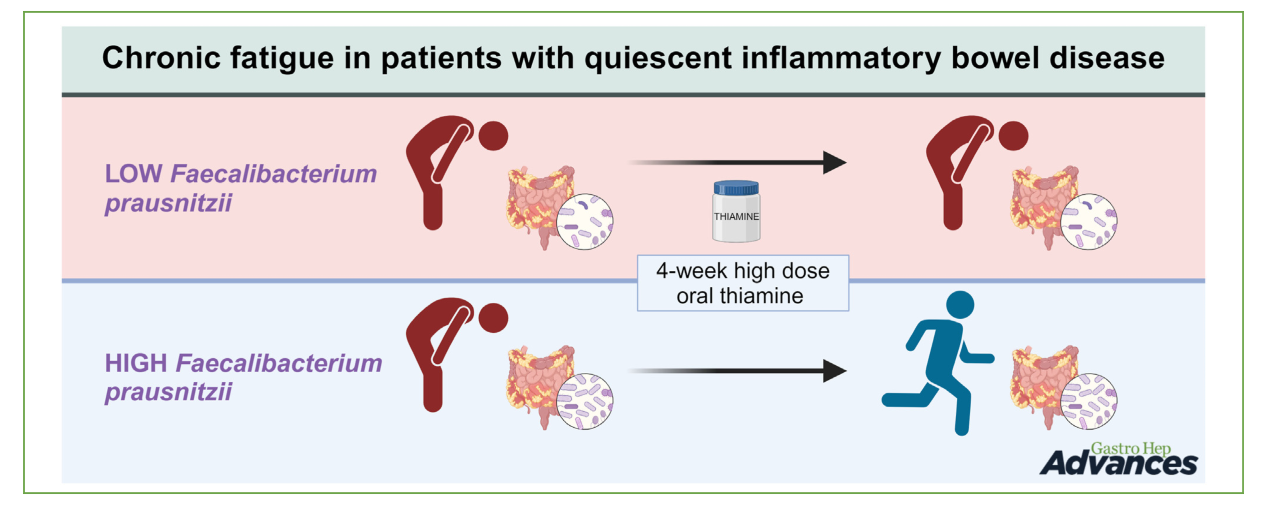
Thiamine-Reduced Fatigue in Quiescent Inflammatory Bowel Disease
-
In this study, they compared the microbiome of responders and non-responders to thiamine. They found that the composition of microbiome made a difference.
-
@DavidPS
Thanks for posting but I’d like to reframe…
DavidP says:
“They found that the composition of microbiome made a difference”
=> Yes, but they suggest (thanks to the figure) that HD thiamine has the power to modify the microbiome towards the beneficial Faecalibacterium prausnitzii. It’s not the case. The researchers should have connected the dots in a different way.- A lack of thiamin has an impact on metabolism of some bacteria.
- If helpful bacteria (F. prausnitzii, R. hominis, A. muciniphila) is less present, and Bacteroidetes more present than firmicutes, the microbiome is often upset.
- A thiamine deficit has been linked to neurological pathologies.
- If energy is low, we struggle and recover with difficulty from stress (in a broad sense). We are less resilient. Vicious circle.
Said differently, thiamine improves the situation but it doesn’t change the microbiome. At least, the conditions are on the way to enable a restoration. Less side-effects due to a lack. As a part of the solution.
Excerpt
Borren et al. also found a depleted pathway of butyrate synthesis in patients with chronic fatigue. Since bacterial butyrate synthesis depends on thiamine as a cofactor for the enzyme pyruvate:ferredoxin 2-oxidoreductase,9 we hypothesized that supplementation with this vitamin could enhance microbial butyrate production. Importantly, we did not see an increase of butyrate-producing bacteria. Thiamine supplementation could have increased the Faecalibacterium prausnitzii butyrate production, without increasing its abundance, but fecal metabolite analysis revealed no increase in butyrate concentration following thiamine treatment. One reservation to this finding is that since SCFAs are utilized by the colonocytes, the levels measured in feces may not give the true picture of the microbial production. In mammals, thiamine is necessary for energy metabolism and to maintain nerve cell function. Supplementation has been studied for different fatigue-related conditions, such as exercise-induced fatigue.38 Thiamine was also reported to improve symptoms of reduce chronic fatigue related to mitochondrial myopathy in 1 patient.39 Thiamine turnover is generally incompletely understood in the gut environment, and there is still much to learn about its acquisition by microbes.40 Overall, this study suggests that thiamine’s mechanism of action in chronic fatigue does not involve the microbiota. While a larger relative abundance of Faecalibacterium prausnitzii predicted a positive response to thiamine treatment, we remark that improved fatigue levels were not associated with changes in abundance of Fecalibacterium prausnitzii. (…)
In conclusion, we found no differences in gut microbiota between patients with qIBD with or without chronic fatigue. The structure and stability of the fecal microbiota were not affected by thiamine or associated with improvement of fatigue symptoms. Importantly, the correlation between change in IBD-FQ1 and relative abundance of Faecalibacterium prausnitzii or Roseburia hominis indicated that Faecalibactium prausnitzii or Roseburia hominis might serve as predictors of thiamine treatment effect for chronic fatigue likely because they are markers of a better general gut health in patients who respond to high-dose thiamine. Validation studies conducted in large cohorts are essential to apply these insights in clinical settings.
Comment (LucH)
A 4-week high-oral-dose thiamine regimen (600–1800 mg/d)
Chronic Fatigue was Not Associated with Gut Microbiota Composition
Though the analysis of the relative abundance of specific species associated with chronic fatigue (Faecalibacterium prausnitzii, Ruminococcus species and Roseburia hominis) revealed that Faecalibacterium prausnitzii was significantly more abundant in responders, a supplementation with these phyla didn’t change the response in both groups. The researchers found no differences in gut microbiota between patients with qIBD with or without chronic fatigue. The structure and stability of the fecal microbiota were not affected by thiamine or associated with improvement of fatigue symptoms.
Said differently, thiamine improves the situation but it doesn’t change the microbiome. Note that reduced Akkermansia muciniphila and F. prausnitzii are signs of an unbalance helpful microflora. These are useful in controlling the low-grade inflammation of the bowels.
https://www.sciencedirect.com/science/article/pii/S2772572324001274#:~:text=10,IBD compared with healthy controls.
Composition of the Gut Microbiota
The bacteria in the gut microbiota are divided into different phyla, the two main ones being Firmicutes and Bacteroidetes.
Each individual has a unique microbiota, but certain phyla generally dominate, such as Firmicutes (approximately 60-75%) and Bacteroidetes (approximately 30-40%).
The balance between these different bacteria is crucial for optimal microbiota function. -
@LucH - thanks for your detailed thoughts. I always appreciate your input.
In my twisted interpretation of this study, I considered it to be an additional indication that our present microbiome may be the reason why things (like thiamine) do not work 100% of the time.
People are fond of saying we are all different. "One size does not fit all." Studies like this remind me that we all have different microbiomes which has an impact on our outcomes. Environmental factors such as antibiotics, protein pump inhibitors and the foods we eat change our microbiome.
-
@DavidPS said in Thiamine-Reduced Fatigue in Quiescent Inflammatory Bowel Disease:
I considered it to be an additional indication that our present microbiome may be the reason why things (like thiamine) do not work 100% of the time.
Well seen. I agree: Thiamine is part of the solution. When eating carbs (pasta rice & bread), we lack 50 % of the required B1.
Moreover, there are often minimum 2 additional problems in such a context (unbalance of the microbiome): low energy level and inflammation, impacting general metabolism.
Expressed differently:
I'd cherish Akkermansia Municipalis, but only after correcting the balance. For SFA.
And I'd target transit to avoid stagnation.
Afterwards Thiamine 100 mg + some B2 2x/wk. (...). -
I take 180mg of ttfd once a week
on the day I take it I get intensely high level circulation (more redness in fingers), higher warmth, general overall feeling of more wellness, more relaxation and the mood boost
I feel good all other times anyways but it is like taking a drug
kind of the same way I user 325mg aspirin in baking soda once in awhile
taking these things too frequently seems to lessen the feel-good effect, for I.
-
I've been taking about 1000mg per day of thiamine HCl and 50mg of alithiamine for maybe 2 years.
My gut health has never been better. I can tolerate almost any food now that I used to have problems with.
I used to have constant blood on the toilet paper, for 20 years. It's been years since I had that issue.
Is it the thiamine? Maybe.
It also helps me breathe better because it raises CO2 levels.
-
Maybe that's why every type of B1 causes hair loss for me...
Taking vitamin B2 increases F. Prausnitzii . So B2 could help with B1 tolerance.
Just as B2 helps to convert B6 into its active form. Another reason to take a B-complex. -
@DavidPS How do we improve our microbiome?
-
@heyman said in Thiamine-Reduced Fatigue in Quiescent Inflammatory Bowel Disease:
How do we improve our microbiome?
Vary. Not always eating the same kind of food.
Useful info
Dominant Species
Excerpt from this post: “Impact du Microbiome”
http://mirzoune-ciboulette.forumactif.org/t1589-impact-du-microbiome#18869
• 80% of our immunity depends on interaction with intestinal bacteria.
• Bacteria live in symbiosis with the host as long as a species—a phylum—does not seek to expand its range…
• Species diversity is important.
• The two dominant phyla are Bacteroides and Firmicutes. They produce beneficial SCFAs, mainly from polysaccharides derived from indigestible fiber.
SCFA = Short-Chain Fatty Acids
• A diet with or without fiber, in appropriate quantities (20–30 g), will influence the dominant type of bacteria.
• The type of microbiota affects nutrient acquisition (assimilation), energy recovery (calories extracted) and host metabolic pathways (hormones and immunity).
• Short-chain fatty acids (SCFAs) are produced mainly from resistant starches and polysaccharides, thanks to the action of certain bacteria. We need SCFAs and CLA to produce certain hormones important for the integrity of the intestinal barrier.
The microbiota is composed of 90% strains of the Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes phyla. Firmicutes (60–80%); Bacteroidetes (15–30%). The Firmicutes phylum/type encompasses over 250 genera, including Lactobacillus and Clostridium, while the Bacteroidetes phylum/type comprises approximately 20 genera. The most abundant phylum is therefore the Bacteroidetes phylum. Both Firmicutes and Bacteroidetes phyla produce beneficial SCFAs from indigestible carbohydrates (e.g., fiber) that reach the colon; Firmicutes being the primary butyrate producer and Bacteroidetes producing mainly acetate and propionate. (2)
SCFA = Short-Chain Fatty Acids (Coconut butter, for example, contains +/- 66% SCFA, and 90% Saturated Fat).
Figure: Proportions of bacteria (diet with or without fibers)
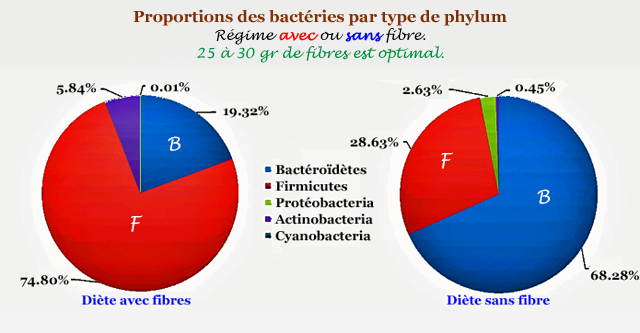
Decoder:- In red (left), people eating fibers (veggies). 30 g / day.
- In blue (right), diet without much fiber.
NB: You won't get the same microbiota with or without meat. Need polyphenols to balance.
If > 55 % carbs from bread / pasta / rice / potato / snack, there will be a distortion. Most of the time after taking antibiotics or if constipated.
-
@heyman Easier said than done but improving the secretion of antimicrobial peptides in the gut should be an important step.
-
@heyman said in Thiamine-Reduced Fatigue in Quiescent Inflammatory Bowel Disease:
@DavidPS How do we improve our microbiome?
Focus on eating a diverse range of plant-based foods, which are rich in fiber and polyphenols that support good gut bacteria.
There is a lot of suggestions on the internet. Find several suggestions and then look for common elements. @LucH has given you a great place to start. Here are 2 links to hel you get started.
From the BBC - 15 tips to boost your gut microbiome
-
Ai
Key Strategies to Improve Your Microbiome
1. Increase Dietary Fiber
Benefits: Fiber acts as a prebiotic, feeding beneficial gut bacteria.
Sources: Aim for 21 to 38 grams of fiber daily from:
Legumes (beans, lentils)
Whole grains (oats, quinoa)
Fruits (berries, apples)
Vegetables (leafy greens, sweet potatoes)
Nuts and seeds
>2. Stay Hydrated
Importance: Water supports digestion and nutrient absorption.
Recommendation: Drink enough water to prevent dehydration and maintain gut health.
>3. Incorporate Fermented Foods
Examples: Yogurt, kefir, sauerkraut, kimchi, and kombucha.
Benefits: These foods contain probiotics, which can enhance gut diversity.
>4. Limit Processed Foods
Impact: Ultra-processed foods can negatively affect gut health.
Advice: Focus on whole, unprocessed foods to support a healthy microbiome.
>5. Manage Stress
Connection: Stress can disrupt gut health.
Techniques: Practice relaxation methods like meditation, yoga, or deep breathing.
>6. Regular Exercise
Effect: Physical activity promotes a diverse microbiome.
Goal: Aim for at least 150 minutes of moderate exercise weekly.
>7. Get Enough Sleep
Recommendation: Aim for 7 to 9 hours of quality sleep each night.
Benefit: Good sleep supports overall health, including gut health.
Conclusion
Improving your microbiome involves a combination of dietary changes, hydration, stress management, exercise, and sleep. By adopting these practices, you can enhance your gut health and overall well-being.