H. pylori, one of the worst bacteria of all
-
https://sci-hub.se/10.1007/s00253-021-11165-6
"Emerging evidence suggests that Helicobacter pylori infection is associated with metabolic disorders, although the underlying
mechanisms are poorly defined. This study aimed to investigate the interaction among H. pylori, a high-fat diet (HFD), and the
gut microbiota with glucose regulation and alterations in microbial metabolites. Mice were randomly allocated to H. pylori infected and noninfected groups fed a chow diet or an HFD. After 4 weeks, two of the HFD groups were given antibiotic cocktails
for 8 weeks to eliminate the gut microbiota. The results showed that an HFD significantly promoted increases in body weight,
insulin resistance, and glucose intolerance, which were alleviated to normal after antibiotic treatment. H. pylori infection
aggravated HFD-induced hyperglycemia, which could not be restored by antibiotics. The perturbation of the gut microbiota
was greater in the mice cotreated with H. pylori and an HFD (HFDHp) compared to those administered either H. pylori or an
HFD alone, with a loss of diversity, higher abundance of Helicobacter, and lower abundance of Lactobacillus. Furthermore,
compared to that of the HFD alone group, the gut microbiota of the HFDHp group was much more susceptible to antibiotic
destruction, with extremely lower diversity and dominance of Klebsiella. Fecal metabolome analyses demonstrated that the
combination of H. pylori infection and an HFD altered metabolic composition and function, which were linked to glucose
dysregulation. H. pylori infection may exacerbate the dysbiosis of the gut microenvironment induced by an HFD, including
alterations in the microbiota and metabolites, which weakens the restorative effect of antibiotics and results in the persistence of
glucose disorders."Next to that H Pylori causes:
- a constant ammonia production
- makes enzymse that degrade carbon dioxide
- it thrives on glucose, so it can explain if you get inflammation from sugar and little of the benefits
- makes stomach acid weak, so that you won't digest proteins well
- this stomach acid is also the main defense mechanisms to make sure the stomach and small intestines stay sterile, so increased risk of SIBO
- causes overgrowth of bad bacteria in your colon (proteobacteria, adhesive escherichia coli, etc)
- downregulates your vitamin D
I also don't think Ray Peat inspired diets are gonna fix this, but actually could make it worse.
Natural stuff that can eradicate it in some cases:
- Black cumin seeds (3x 2g a day) and honey (3x 6g a day)
- Cranberry juice
-
@Kasper, why do you think so?
I also don't think Ray Peat inspired diets are gonna fix this, but actually could make it worse
Also, could you please provide a pubmed or other 'official' site link?
The sub-hub.se doesn't display on all browsers/set-ups equally well.
(I get a blank page, e.g.)
Thank you -
I find that swallowing a tablespoon or so of honey when I wake up and nothing else for an hour gets rid of them, doing this for several mornings every few weeks.
I can tell this works because when I use aspirin it only hurts my stomach when H Pylori is infesting.
-
The carrot salad should help with honey and I add aronia berry(chokeberry) too it as well and it has similar properties to cranberries
-
@Lejeboca said in H. pylori, one of the worst bacteria of all:
I also don't think Ray Peat inspired diets are gonna fix this, but actually could make it worse
I suspect that mostly fruit juices and plain sugar could exacerbate it. Sugar works best with little bacteria in your stomach and small intestine.
Ray Peat used sugar to calm down inflammation, but if it exacerbates inflammation because Pylori feeds on it, then I think the net effect is negative.
In my personal experience, whole fruits (including the fiber) and carrots work better in those cases as source of carbs.
For example, pectin has anti pylori activity:
https://www.scirp.org/html/3-7300424_28439.htm@Lejeboca said in H. pylori, one of the worst bacteria of all:
The sub-hub.se doesn't display on all browsers/set-ups equally well.
Here is the link:
https://pubmed.ncbi.nlm.nih.gov/33576881/ -
And yet, people try to find the bright side of it through associations:
"Despite well-documented carcinogenic properties, H. pylori infection is pervasive both through time and across space. It is therefore possible that the bacterium confers a benefit to its host under some circumstances and is therefore maintained in the human population. Some evidence for such a benefit exists (Table 1); it has been suggested that H. pylori confers protection against tuberculosis (TB) through induction of antagonistic interferons for the causative agent, Mycobacterium tuberculosis, and that it plays a role in reducing the risk of esophageal adenocarcinoma (EAC), gastroesophageal reflux disease, stroke, lung cancer, asthma, allergies, and irritable bowel disease (Blaser 1999; Ahmed et al. 2009; Luther et al. 2010; Perry et al. 2010; Plottel and Blaser 2011; Walker and Talley 2014). Antibacterial activity against other virulent bacterial species has also been documented, showing a preventative effect against diarrheal diseases and an augmented enteral and systemic response to Vibrio cholerae, the causative agent of the disease cholera (Blaser and Berg 2001; Cohen et al. 2012; but see Clemens et al. 1995; Shahinian et al. 2000)."
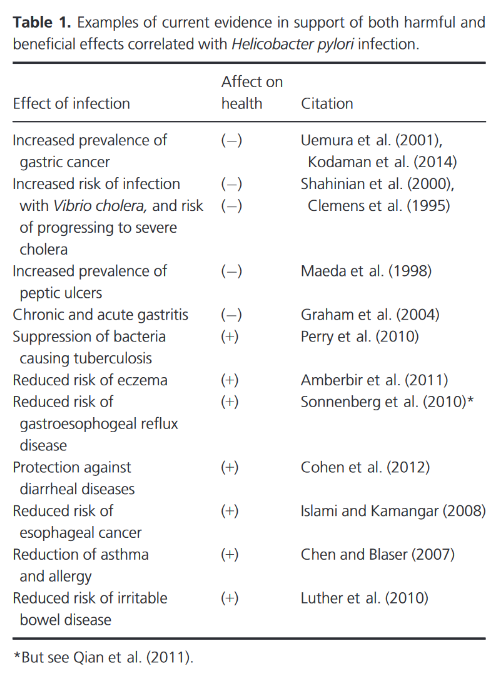
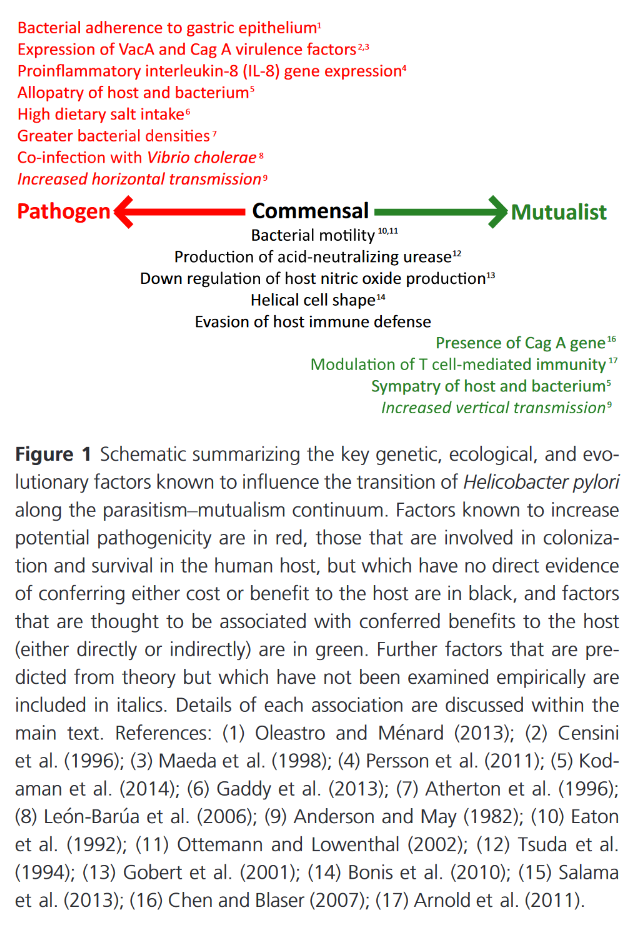
-
Helicobacter pylori arginase mutant colonizes arginase II knockout mice
"Urease is the most abundant protein in H. pylori, and accounts for up to 10% of the total cellular protein[5]. It is assumed that urea, the substrate for urease, must be provided abundantly in vivo from either the bacterium or the host in order for urease to function efficiently (Figure 1). Indeed, the in vivo concentration of urea in the stomach is approximately 5 mmol/L[12,13], which is well above the Km for urease, and yet the in vivo source of the urea for H. pylori urease has remained unknown. It originally had been hypothesized that the urea comes from the bacterial arginase, RocF. Arginase catalyzes the hydrolysis of arginine to ornithine and urea (Figure 1). The surprising finding that arginase (rocF) mutants of H. pylori can still colonize mice suggests that another in vivo source of urea must exist[14]. The urea may come from direct release from gastric epithelial cells or other cells in gastric pits lining the stomach. Alternatively, the urea may diffuse into the gastric juice from the bloodstream. In either case, host arginases would be responsible."
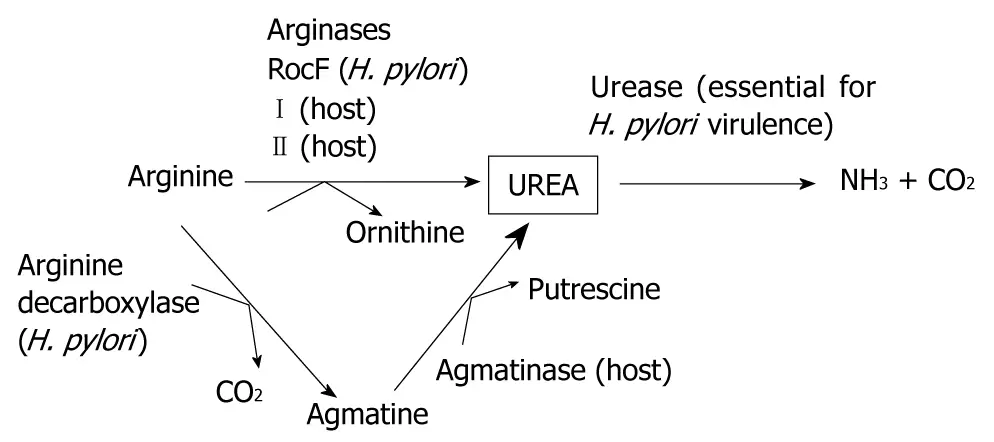
"There are two known host arginases: arginase I and arginase II. Arginase I is the cytoplasmic enzyme that is expressed heavily in the liver and at lower levels in a few other tissues, whereas arginase II is the mitochondrion-associated enzyme that is expressed in many tissues and cells, including the stomach, kidneys and macrophages[15]."
"Arginase II is expressed in the human and mouse stomach[16], therefore, we reasoned that the arginase II knockout mouse would allow us to determine the relative contributions of host versus bacterial arginase to colonization in vivo, through the production of urea."
"We demonstrated that the H. pylori rocF mutant, which has no detectable arginase activity, colonized host arginase II knockout mice. These data are very surprising, because host arginase II is expressed in the stomach, and is even upregulated by H. pylori infection[16]. Moreover, inhibiting at least some of the host arginase I by BEC treatment failed to block the ability of the rocF mutant to colonize arginase II knockout mice. The data suggest that H. pylori must obtain its urea from either the residual arginase I that is not inhibitable by BEC, or an alternative pathway of host urea generation, such as agmatinase. The data also suggest that enzymatically active host arginase I occurs in the stomach, as has also been suggested previously[28]."
"The abundant urease must obtain copious amounts of urea for the urease to function efficiently in vivo, since urease is absolutely essential for colonization[8,9]."
"Agmatine is prevalent in the stomach[35,36] and 60% of this agmatine goes to the liver[37]. Interestingly, agmatine levels are higher in the gastric juice of H. pylori-infected patients versus uninfected patients[36]. Moreover, H. pylori has a putative arginine decarboxylase that could theoretically generate the agmatine. There is also the possibility that intestinal normal flora bacteria could provide the urea[38]."
"The in vivo source of urea still remains a mystery. However, it is clear that the in vivo source of urea for the crucial enzyme urease does not originate from the bacterial arginase or from host arginase II. Instead, the data suggest that urea originates from host arginase I or an alternative urea-generating pathway."
-
I remember reading somewhere that apple cider vinegar with each meal may get rid of it. There are ACV capsules, more convenient. I bet coconut oil with its antimicrobial properties would help too.
Ray Peat says the stomach and small intestine should be sterile so I bet he would encourage trying to get rid of h. pylori
-
Can anyone please send me a link where Peat talks about h pylori? I want to read his words/views on it. His website is hard to use,with no search bar, etc. Maybe there's no info online but rather in a book by him?
Thanks -
Small amounts of mastic gum can eradicate h pylori.
The traditional way of using it is to chew it and not just swallow capsules. I believe this works better because it also removes it from the oral cavity where it may hide deep in the gums. Its relatively cheap to try if you are having any stomach issues. -
@Homebythesea
https://youtube.com/watch?v=5sop_zLMZ34
1:01:26 onwards -
That study doesn't describe the composition of the high-fat diet.
It's likely all soy and sunflower oils etc? Then the negative effects of HFD on H.p. wouldn't be surprising.
I've recently read that butyrate or beta-OH-butyrate supports H.p. eradication. It's by epigenetic restimulation and restoration of the gastric mucosa so that it overcomes and more easily rejects the altering influence of the occupying bacteria (which also take on resilient, viable but not culturable spore forms inside the gastric crypts, btw, to survive antibiotic or environmental insults). Pretty sure that But or bOHBut are not part of HF lab chow but be even lower on those diets due to lack of fiber fermentation. -
if you literally unironically
have h pylori literally unironically you could do the sprouted and frozen for 48/72 hrs brocolli sprout smoothies. it’s not peaty (le cruciferous… is …. le bad!!!) yet i think it works. the sulforaphane content like this from 15g of seeds is more than comes in expensive supplements.you could eat raw red cabbage (the spicier the cabbage the better, and i’ve heard organic raw red cabbage is the spiciest) for the same type of effect just at a lower dose. this also has sulforaphane but not as much as the brocolli sprouts. might be milder.
other than that honey and coconut oil apparently works too.
-
"Green tea inhibits Helicobacter growth in vivo and in vitro"
https://pmc.ncbi.nlm.nih.gov/articles/PMC2694061/Mice were infected with H. pylori and given either tea or water. The group that was given tea had both greatly reduced inflammation and H. pylori population. In fact, mice that were given tea both before and after the infection had no inflammation and no H. pylori population at all.
I suspect it is the L-Theanine in the tea but nobody has studied that so I have no proof. I think so because high dose L-Theanine completely suppresses H. pylori symptoms for me.