Phosphorus, the toxin
-
More toxins on the scene. What are the remaining nutrients? Magnesium?
- Phosphate is a vascular toxin
- Phosphate Is a Uremic Toxin
- Phosphate Is a Cardiovascular Toxin
- Dietary phosphorus as a nutritional toxin: the influence of age and sex
The outer shell of phosphorus contains 5 electrons.
When we connect them, the message is clear.

What authorities are trying to accomplish by recommending its consumption?
- Scientific Opinion on Dietary Reference Values for Calcium | EFSA
- Scientific Opinion on Dietary Reference Values for Phosphorus | EFSA
- Phosphorus | Institute of Medicine
- The Institute of Medicine’s “Dietary Reference Intake” for Phosphorus: A Critical Perspective
Absorption
The absorption rates of calcium and phosphorus are not proportional:
Disorders of Calcium, Phosphate, and Magnesium Metabolism
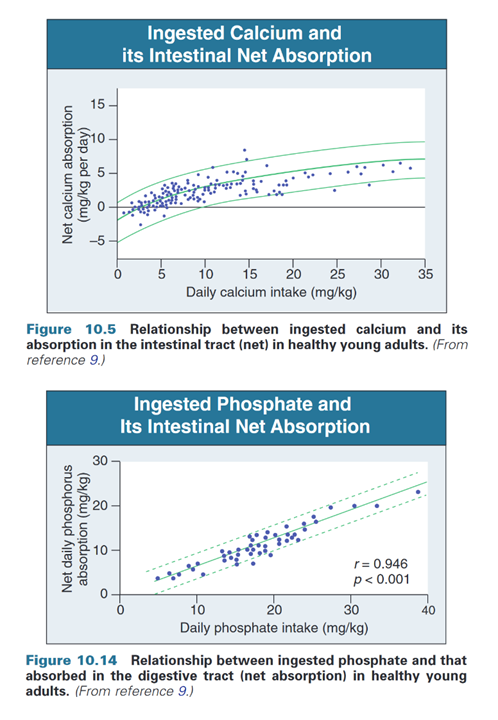
"The amount of dietary calcium intake [..] regulates the proportion of calcium absorbed through the gastrointestinal tract (Fig. 10.05)."
"Absorption is a linear, nonsaturable function of phosphate intake (Fig. 10.14) and amounts to 60% to 75% of total phosphate intake (15 to 50 mmol/day)."
But within reasonable amounts, the absorption fraction may be simplified as:
Adults
- Ca: 30%
- P: 60%
- Ca 1:2 P
- Ca: 60%
- P: 80%
- Ca 1:1.3 P
Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease
"Inorganic P, such as P additives, are not protein bound; they are salts that more readily disassociate and are absorbed in the intestinal tract (50). Indeed, it is believed that >90% of inorganic P may be absorbed in the intestinal tract, as opposed to only 40 to 60% of the organic P present in natural foods (51,52). The major public health implication from these considerations is that the P burden from inorganic P–containing food additives is disproportionately high relative to organic P. In the early 1990s, P additives contributed approximately 500 mg/d P to the American diet, whereas today P additives may contribute as much as 1000 mg/d P to the average American diet (37,51,53,54)."
Twenty-Four-Hour Urine Phosphorus as a Biomarker of Dietary Phosphorus Intake and Absorption in CKD
"Accurate and reliable assessment of phosphorus intake is needed, but this is complicated by limitations in the tools available (9). These limitations include the nutrient databases, which are incomplete and often inaccurate for phosphorus. Differences between phosphorus content determined by nutrient database versus direct chemical analysis of foods show that databases can drastically underestimate phosphorus content of foods (approximately 15%–70%) [?] (10–16)."
Calcium and phosphorus interaction in the gut
Phosphorus Nutrition and the Treatment of Osteoporosis
"Food phosphorus binding by coingested calcium is a well-recognized and well-used approach, particularly in the management of phosphorus absorption in patients with end-stage renal disease.” “Heaney and Nordin[15] have shown that fecal phosphorus content (and therefore, inversely, absorbed phosphorus) is determined primarily by unabsorbed calcium and to a lesser extent by phosphorus intake. Together, dietary calcium and phosphorus intakes account for nearly three fourths of the variation in the quantity of phosphorus absorbed. Briefly, each 500 mg of ingested calcium binds 166 mg of diet phosphorus (95% confidence interval, 144-188 mg)."
"The degree of interference reported here (~166 mg phosphorus for every 500 mg ingested calcium—0.4 mmol phosphorus for every mmol calcium) is substantially less than might have been predicted from simple stoichiometry. A 1:1 molar ratio for CaHPO4 would predict binding of 388 mg phosphorus by 500 mg calcium and, for Ca3(PO4)2 at a Ca:P molar ratio of 1.5:1.0, 258 mg phosphorus would be bound per 500 mg calcium. A molar ratio of 1:1 seems more likely at digestate pH values below 7.0. Even at the upper end of the confidence interval for the regression model—210 mg (6.8 mmol)— binding would still be substantially less than the lowest stoichiometric prediction. This discrepancy may be partly due to rapid absorption of phosphorus in the duodenum well before calcium and phosphorus complexes can form in the chyme. In any event it undoubtedly reflects the complexity and multiplicity of the interactions within the digestive residue." (10.1080/07315724.2002.10719216)
"Under such circumstances, absorbed phosphorus may be too low to support both soft tissue phosphorus needs and new bone mineralization (which consumes mineral at a Ca:P molar ratio of ~1.6:1). Any induced phosphorus insufficiency under these circumstances, expressed as a lowering of serum inorganic phosphorus concentration, would not only limit bone gain, but enhance osteoclastic bone resorption [5]—precisely opposite to the direction toward which current anti-osteoporosis therapy is pointed. Moreover, teriparatide, specifically, exerting its inherent parathyroid hormone activity [↑PTH; ↓Pi], would itself lower the renal phosphorus threshold, thereby compounding the problem by lowering serum inorganic phosphorus levels still further." (10.1080/07315724.2002.10719216)
"Using this relationship, Figure 2 shows the amount of food phosphorus that would be available for absorption in a woman ingesting 70% [490 mg] of the phosphorus RDA [700 mg] (
Table 1) under varying conditions of dietary and calcium supplementation. The calculations used in constructing Figure 2 assume that the calcium supplements are coingested with the food phosphorus.[15] At a calcium supplement intake of between 1000 and 1500 mg/d, all the food phosphorus is complexed by unabsorbed calcium and rendered nonavailable. Above that point, phosphorus absorption becomes negative (ie, phosphorus in digestive secretions and sloughed intestinal mucosa, estimated at as much as 250 mg/d,[16] will be complexed as well, blocking its reclamation by the gut; this converts the gut into a net excretory organ for phosphorus)."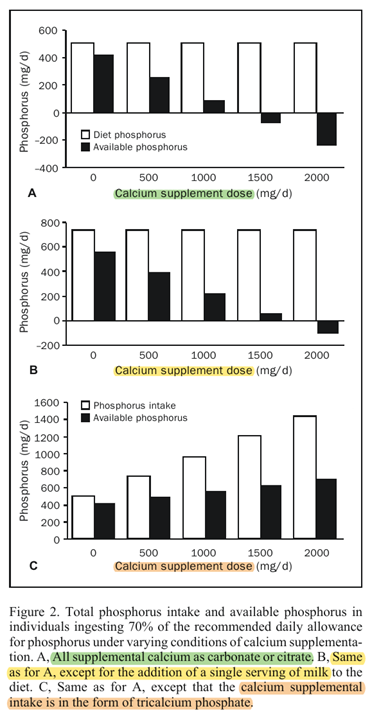
"Adding a single serving of milk to the diet of such women eliminates much of the problem. Alternatively, the use of a calcium phosphate supplement provides the desired calcium, prevents the blocking of food phosphorus absorption, and ensures a generous supply of available phosphorus. Although total phosphorus intake rises nearly 3-fold with the highest of the calcium phosphate supplement scenarios, available phosphorus rises very little. Thus, basically what the calcium phosphate supplement does, in addition to providing the desired calcium, is to spare food phosphorus."
"The timing of the calcium supplement intake is important for this relationship. As noted, the foregoing calculations are for simultaneous ingestion of calcium and phosphorus, which is in accord with the usual recommendation. Taking supplements with meals remains a sound stratagem because by slowing gastric emptying, the meal effect improves calcium utilization efficiency, and by spreading the dose throughout the day, calcium absorption is improved further. However, this same stratagem maximizes phosphorus binding and minimizes phosphorus absorption. Schiller et al[17] have shown, using a single-meal design, that separation of the calcium supplement dose from the time of the phosphorus-containing meal substantially reduces the degree of phosphorus binding. This is, of course, to be expected for a process that involves nothing more complex than simple chemical binding. Figure 3 plots some of the data from the article by Schiller et al, showing both the degree of reduction in phosphorus absorption produced by simultaneous coingestion of calcium and phosphorus and the reduction in this effect produced by giving the calcium supplement 2 hours after the meal."
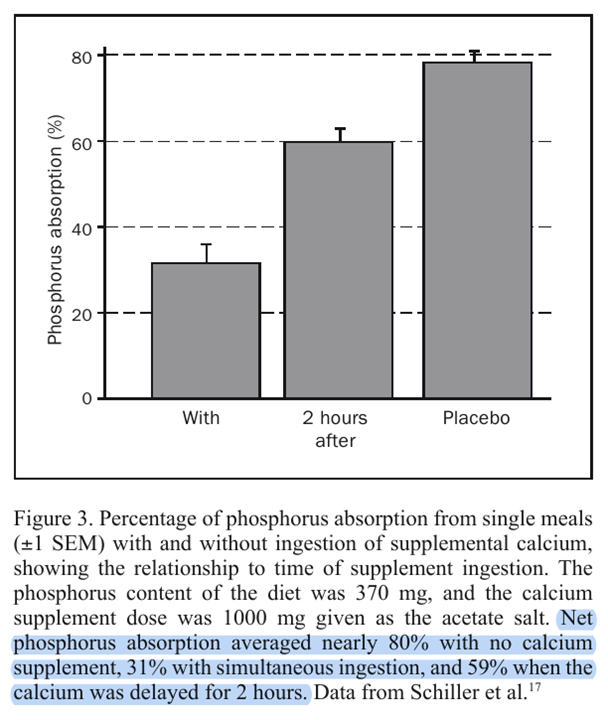
Consequences of Phosphate Imbalance
"Phosphate is absorbed all along the intestinal tract, with the jejunum being the most active absorptive site (91, 184). It is excreted into the gastrointestinal tract with the digestive juices in the amount of approximately 200 mg/day (193), about two thirds of which is reabsorbed (153). Saliva and bile acids are the most important gastrointestinal secretory fluids of phosphate (194)."
"The relative amounts of Ca and P in the intestinal lumen may have another important implication. It has been hypothesized (134, 187, 188) that ionic calcium in the colonic lumen forms nonsoluble complexes with free bile acids and fatty acids. By forming these complexes, calcium can protect the colonic mucosa from the damaging effects of these acids. Free bile acids and fatty acids are thought to enhance cellular profileration in the colonic mucosa (27, 109), a process that can lead to neoplastic changes and eventually to cancer. In the presence of high concentrations of phosphate in the intestinal lumen, calcium becomes bound to phosphate in nonsoluble complexes; this decreases the amount of ionized calcium available for neutralization of free bile and fatty acids and their damaging effect on the mucosa." "Low sodium in the intestinal lumen reduces phosphate absorption (124), while high K increases it (69, 174)."
Circulation and Distribution
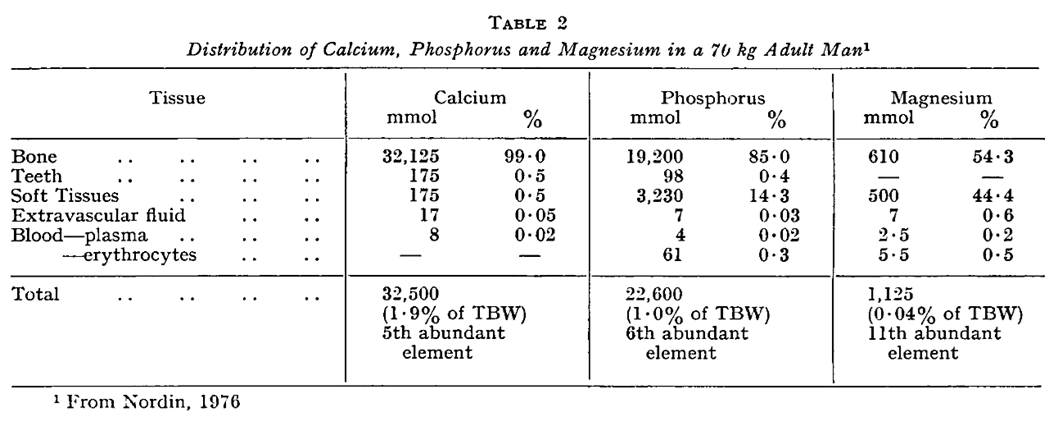
Calcium, Phosphorus and Magnesium Turnover
Consequences of Phosphate Imbalance
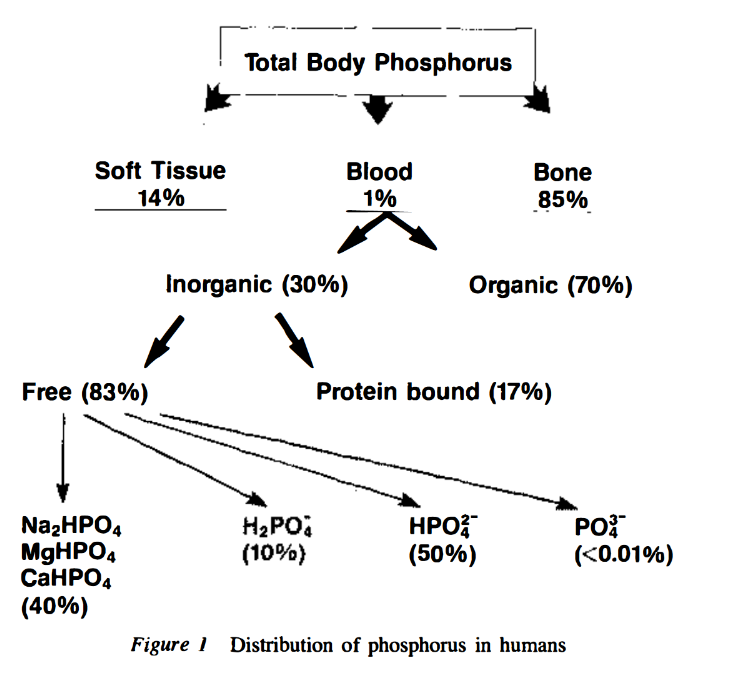
Role of transporters in regulating mammalian intracellular inorganic phosphate
"[..]physiological [Pi]in, in many but not all mammalian cells, is about 1-5 mM."
"A partial list of published MRS data on [Pi]in (
Table 1, upper) shows that in mammalian cells other than erythrocytes, [Pi]in is generally equal to or higher than [Pi]ex. The MRS data indicate that [Pi]in under resting conditions is ∼4 mM in skeletal muscle (highest in slow-twitch fibers) and ∼1 mM in the heart and brain."Phosphorus-contaminated molecules
For the 14% of phosphorus found outside of bones, these are some of the phosphorus-contaminated molecules (and their formula, taken from PubChem):
- TDP (TPP) (C12H19N4O7P2S+)
- FMN (C17H21N4O9P) and FAD (C27H33N9O15P2)
- NAD (C21H28N7O14P2+) and NADP (C21H28N7O17P3)
- CoA (C21H36N7O16P3S)
- PLP (C8H10NO6P)
- Biotinyl '5-AMP (C20H28N7O9PS)
- AdoCbl (C72H100CoN18O17P–3) and MeCbl (C63H91CoN13O14P–3)
- Nucleotide
"Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine, and thymine; in RNA, uracil is used in place of thymine."
"Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP)—throughout the cell for the many cellular functions that demand energy, including: amino acid, protein and cell membrane synthesis, moving the cell and cell parts (both internally and intercellularly), cell division, etc..[2] In addition, nucleotides participate in cell signaling (cyclic guanosine monophosphate or cGMP and cyclic adenosine monophosphate or cAMP) and are incorporated into important cofactors of enzymatic reactions (e.g., coenzyme A, FAD, FMN, NAD, and NADP)." Nucleotide | Wikipedia
- Phosphocreatine
- Phospholipids
- Inositol phosphate
"Inositol phosphates play a crucial role in various signal transduction pathways responsible for cell growth and differentiation, apoptosis, DNA repair, RNA export, regeneration of ATP and more."
"D-2,3-BPG is present in human red blood cells (RBC; erythrocyte) at approximately 5 mmol/L. It binds with greater affinity to deoxygenated hemoglobin (e.g., when the red blood cell is near respiring tissue) than it does to oxygenated hemoglobin (e.g., in the lungs) due to conformational differences: 2,3-BPG (with an estimated size of about 9 Å) fits in the deoxygenated hemoglobin conformation (with an 11-Angstrom pocket), but not as well in the oxygenated conformation (5 Angstroms). It interacts with deoxygenated hemoglobin beta subunits and decreases the affinity for oxygen and allosterically promotes the release of the remaining oxygen molecules bound to the hemoglobin. Therefore, it enhances the ability of RBCs to release oxygen near tissues that need it most. 2,3-BPG is thus an allosteric effector."
"Approximately 13,000 human proteins have sites that are phosphorylated."
Consequences of Phosphate Imbalance
"The phosphate is needed inside the cells for phosphorylation of glucose and fructose and for ATP synthesis. It is well established that glucose load (as given in the glucose tolerance test) induces a transient reduction in the serum phosphate levels (42, 78). In feeding after starvation, hypophosphatemia can occur when insufficient phosphate is given. Again, the carbohydrate load given during feeding causes phosphate shifts into the cells, resulting in hypophosphatemia." "Glycolysis enhances utilization of phosphate and thus induces shifts from the extracellular fluid into the cell."
"Intracellular phosphate regulates the activity of various enzymes affecting (a) glucose metabolism: hexokinase, phosphofructokinase (98, 180); (b) amino acids: glutaminase (137); (c) nucleic acids: AMP deaminanse, 5'-nucleotidase, adenine denucleotidase (58, 93, 193); and (d) hormones: 25-OH-D hydroxylase (195)."
Healthy Nutrition And The Human Musculoskeletal System
Main forms in bone:
Ca:P Synthetic hydroxyapatite 2.2:1 Natural hydroxyapatite 1.9:1 Amorphous (non-crystalline) calcium phosphate 1.7:1 Their mass ratios:
Ca:P Range Bone (measured) 2.2:1 (2.1-2.3):1 Whole-body (calculated*) 1.9:1 (1.8-2.0):1 Whole-body  (measured)
(measured)2.1:1 (1.9-2.2):1 Whole-body  (measured)
(measured)2.2:1 (2.0-2.4):1 *Calculated from bone, containing 99% of body Ca and 85% of body P.
By the way, the EFSA concluded that there are no reliable 'biomarkers of intake or status' and chose their lowest ratio (body composition) to use as reference for intake recommendations:
- Ca (1.8):1 P
- Ca 1:(0.55) P
The recommendation for kilcium is 1000 mg/day, leaving phosphorus at 550 mg/day.
As a side note, they mentioned that the inner bone structure is responsible for most of the turnover in spite of representing only 20% of the total bone mass.
Presentation #552381 | SlideShare
Serum levels
- Calcium: 2.1–2.6 mmol/L (8.5–10.5 mg/dL)
Phosphate- Phosphorus: 0.75 and 1.45 mmol/L (2.5–4.5 mg/dL)
Elimination
"Kidneys filter 180-200 liters of blood per day and each kidney contains approximately 1 million nephrons, which are often referred to as the ‘functional units’ of the kidney." (10.1016/j.pharmthera.2023.108481)
The kidneys recover multiple grams of these toxins a day from blood filtration. The values vary depending on the source, but it's something like 8-10 g of calcium and 4-6 g of phosphate filtered daily.
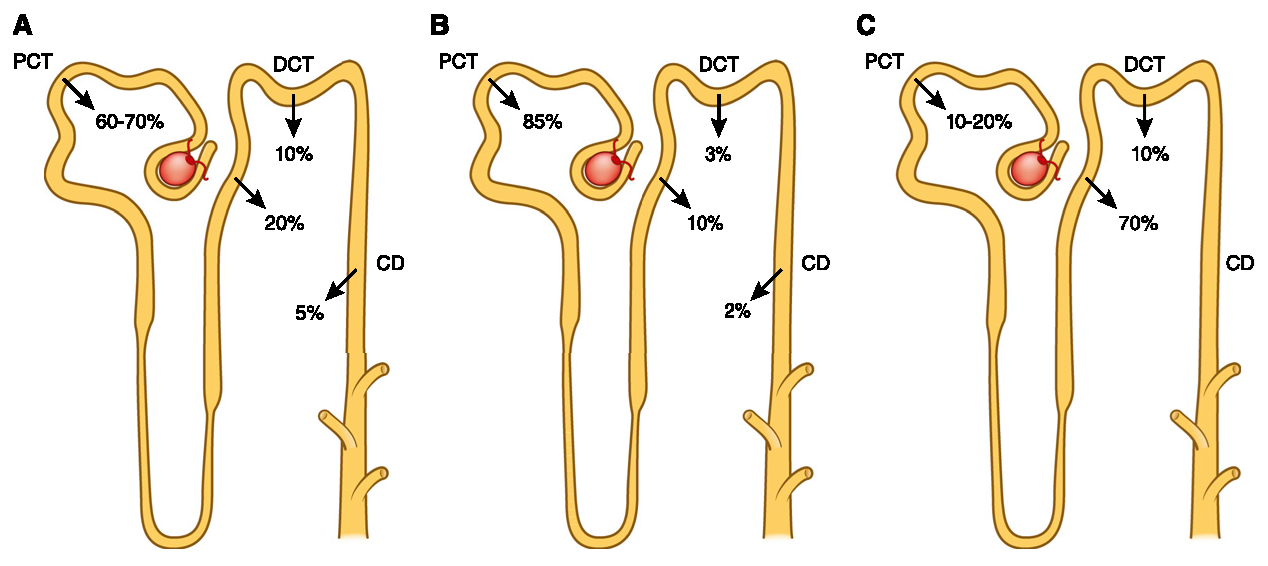
(10.2215/CJN.09750913)Each pass is a chance to make adjustments and eliminate the unwanted fraction, but with limitations. These authors pointed out that the urinary excretion rate is 45 mg/h (can be increased after the effects of PTH or FGF23), and it takes many hours for the absorbed fraction to be entirely eliminated.
Complete absorption is faster than elimination, leading them to speculate that a phosphorus buffer pool exists to maintain stable levels in the blood and inside cells.
Calcium and Phosphorus Metabolism in Chronic Uremia
"It is well known that the physicochemical interrelationships between the two ions concentrations are regulated in such a way that any increase in one is followed by a proportional decrease in the other. This depends on the precipitation of calcium phosphate salts [29]. However, this relationship is not regularly evident in vivo: in fact, the calcium infusion test in normal subjects and in patients with renal osteomalacia is followed by a regular increase in plasma [PO₄] [26,50]. This apparent contradiction to the physicochemical theory may be explained by the phosphate load coming from the cells and compensating in excess for the phosphate delivered to bones [13]."
↳ [13] Renal Excretion of Calcium by the Dog
"Howard (11) explained the phenomenon of increased serum phosphate following intravenous calcium on the basis of a decreased secretion of parathyroid hormone. He suggested that the action of the parathyroid hormone is to alter the ratio between organic and inorganic phosphorus within cells. Thus the primary effect would not be on phosphate excretion but an action increasing the level of circulating phosphate. Whatever the mechanism is for explaining the elevated serum phosphate and decreased urinary phosphate, it must be an immediate effect since in these experiments this phenomena occurred within 10 minutes after the start of the calcium infusion. While it may be dangerous to generalize on phosphorus distribution of total body cells by analysis only of red blood cells, the source of the increased inorganic serum phosphate following intravenous calcium may be assumed to be the organic phosphate esters of the red blood cells."
Calcium and phosphorus balance
I couldn't find the source of the diagrams below, but they give a rough idea of this balance (without showing for phosphorus the buffer pool and losses to the gut):
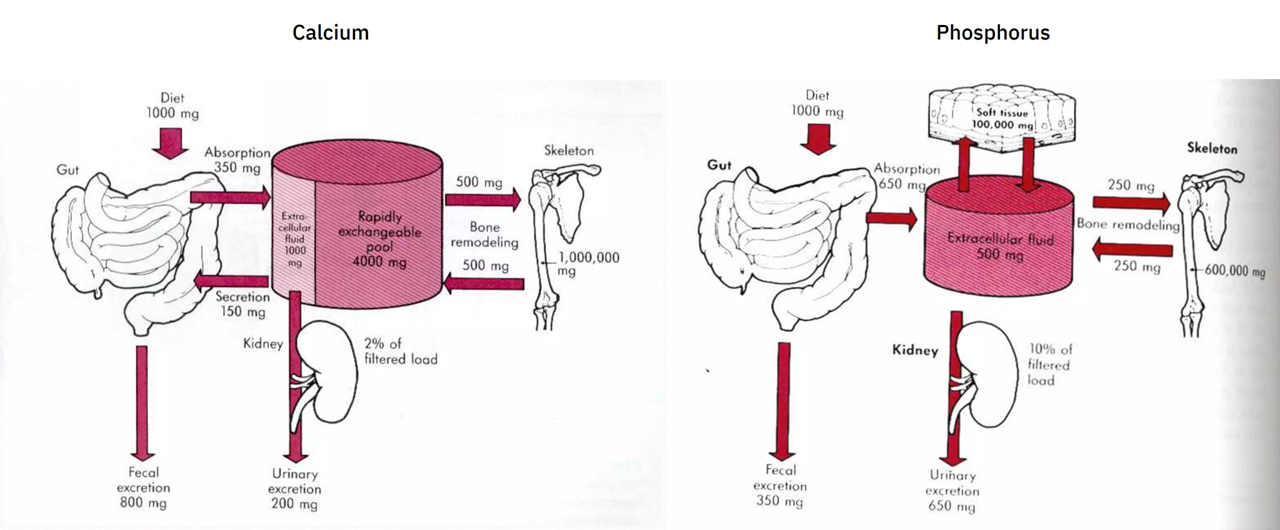
Recall that the approximate absorption ratio is Ca 1:2 P.
The urinary excretion ratio is about Ca 1:3 P (200:650 mg). However, if we lump the amount of calcium secreted into the gut (150 mg; to be eliminated with the unabsorbed portion) with the urinary excretion (200 mg), we arrive on the original absorption ratio of 1:2 (350:650 mg).
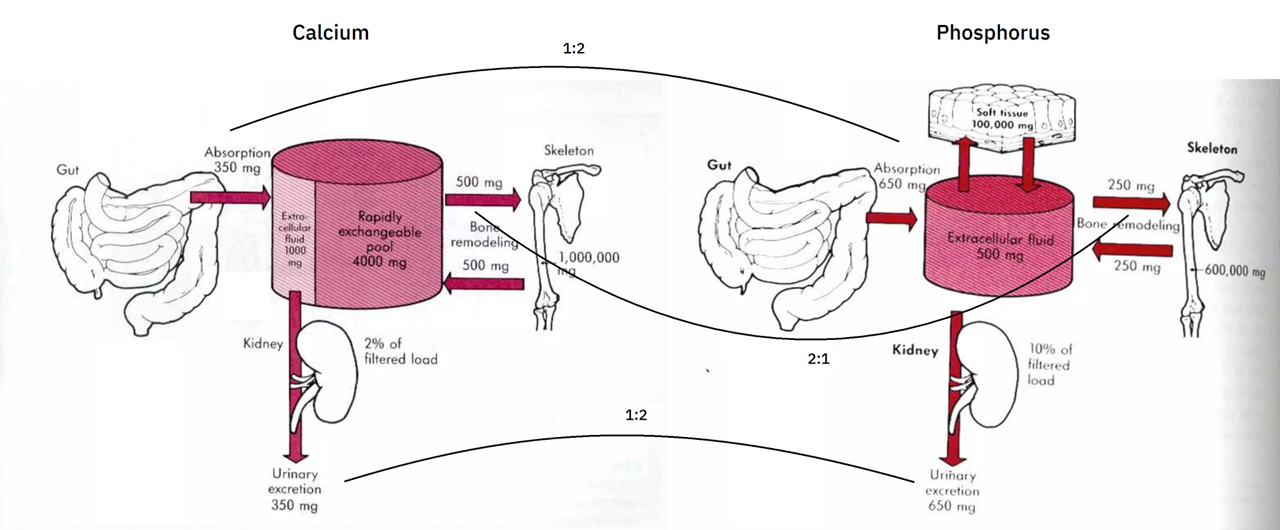
The body absorbs more phosphorus, but the urinary excretion makes up for it. This greater elimination of phosphorus is probably a reflect of absorption rate rather than a fixed property.
Ca:P Absorption/Excretion 1:2 Bone composition/decomposition 2:1 Whole-body (w/ soft tissues) 1.7:1 When tissues are not in equilibrium and the balance is tipped in favor of construction, absorption can be upregulated and excretion downregulated to compensate for the mismatch.
Infants minimize the difference by increasing the intestinal absorption rate of calcium relative to phosphorus, perhaps with further renal retention.
If the next table is right, human milk would have another advantage:
Sipping must also help to prevent saturation and to make adjustments.
Phosphorus Nutrition and the Treatment of Osteoporosis
"Phosphorus is vital for cellular life and activity, being involved in cell structure, information coding, energy transfer, functional activation of catalytic and signaling proteins, and, in short, virtually every aspect of cell function."
"As would be expected for a limiting nutrient, phosphorus absorption by the human intestine is relatively efficient, with net absorption typically ranging from 55% to 80% (depending on concurrent calcium intake and absorption). Moreover, because phosphorus is distributed widely in many foods, it is not likely to function as a limiting nutrient (as is calcium) in animals at the top of the food chain. Nevertheless, circumstances exist in which phosphorus intake may be insufficient to support the bone rebuilding that today may be possible in patients with osteoporosis."
"[..]as can be seen in
Table 1, 5% of women older than 30 years, 10% of women older than 60 years, and 15% of women older than 80 years have intakes on any given day that are less than 70% of the RDA.""[..]the diets of women with low phosphorus intakes are multiply inadequate. The correct solution to this problem is for health professionals first to recognize that the problem exists and then to address it, with either a change in diet or a polyvalent nutritional supplement. Unfortunately, this is not often done in medical practice, and frequently these women, particularly if they have osteoporosis, will be prescribed bone active agents and encouraged to take supplements of calcium and vitamin D."
"Simple maintenance of skeletal mass translates to zero phosphorus balance and places little or no demand on dietary phosphorus intake. This is because urinary phosphorus excretion can be reduced to extremely low levels, and phosphorus released in bone resorption can thus be efficiently reused for mineralization of newly forming bone sites."
"During any age-related decline in bone or muscle mass, phosphorus released in the process can substitute for phosphorus in the diet. Indeed, it must otherwise be excreted by the kidney. Thus, even intakes well below the RDA, whatever other nutritional effects they may have, should be able to sustain the skeletal phosphorus requirements of the typical elderly individual."
"Extremely large calcium supplement doses (eg, >1500 mg/d), particularly if taken regularly as 500 to 600 mg with every meal, could create a negative phosphorus balance situation even for women who are not undergoing antiosteoporosis therapies, particularly if their meat and dairy intakes are low. However, it is improbable that a regimen of invariable coingestion would be rigidly followed. Hence, without a bone-building demand for phosphorus, it is unlikely that phosphorus binding by calcium supplements would be sufficiently complete as to constitute a serious problem. However, once bone active agents, and particularly anabolic agents such as teriparatide, are used, the situation changes.
Figure 2shows that, with calcium carbonate or citrate supplement intakes in excess of 1200 mg/d, there is effectively no diet phosphorus available to support bone building. This supplement dose is less than that used in some teriparatide treatment studies.[18]""In brief, because of the likelihood of some irregularity of the coingestion of calcium supplements with foods, it may still be possible for women with low phosphorus intakes and those receiving antiresorptive therapies to realize the small increase in bone mass that these therapies are able to produce. However, it is unlikely that this would be the case with the anabolic agents, which create a phosphorus demand for bone mineralization that is approximately 10 times that of the antiresorptives. Under such circumstances, it would clearly be prudent to adjust the diet of the patient or to use a calcium phosphate salt as the calcium supplement (or both)."
"Antiresorptive therapies [..] typically produce a slow increase in bone mineral content of 0.5% to 1.0% yearly at the spine after an initial positive remodeling transient of +4.5% to +5.5% in the first year.[14] The steady-state increase in bone mineral content after 1 year creates a small tissue-level phosphorus requirement, with net phosphorus uptake by bone in the range of 4.5 to 9.0 mg/d. By contrast, new-generation anabolic therapies, capable of increasing axial bone mineral content by up to 15% yearly, call for a positive phosphorus balance of up to 45 to 90 mg/d. (The foregoing estimates assume that values measured in the axial skeleton in response to bone active agents apply to the whole skeleton and hence reflect a worst-case, or maximum likely, phosphorus demand.)"
"As reported previously,[15] effective phosphorus deficiency with respect to bone mineralization must express itself as a low value for serum inorganic phosphorus. As long as the bone-forming site is exposed to normal phosphorus concentrations, it will have sufficient phosphorus to support matrix mineralization. In many older individuals with reduced creatinine clearance, there will commonly be some elevation of serum phosphorus levels, which would be protective of the skeleton. The effect of complete or nearly complete food phosphate binding on serum phosphorus in such individuals is unknown. However, they may be protected to some extent. Greater experience with teriparatide therapy will be needed before their relative vulnerability will become clear."
Hormonal regulation of biomineralization
"A very old observation highlights the important role of extracellular phosphate concentration in the mineralization process: by mobilizing phosphate from soft tissue stores, starvation improved rickets in experimental animals[74]. An adequate phosphate level is necessary for normal growth plate development, owing to the phosphate dependent apoptosis of hypertrophic chondrocytes[75]. Infusion of calcium and phosphate in vitamin D deficient rats results in normal mineralization[76]. Furthermore, in a systemic Vdr-knockout model, rickets and osteomalacia can be prevented by a diet rich in calcium and phosphate[66,77]. Similarly, a calcium and phosphate rescue diet improves bone and cartilage in Cyp27b1 knockout mice that lack the 25 hydroxyvitamin D 1α hydroxylase[78]. However, the bone disorders of these animals are not totally normalized by providing minerals, suggesting some possible direct effect of calcitriol as well[79]."
-
Extra information..
Phosphate species distribution
The change in phosphate species as it passes from serum to urine contributes to eliminate H⁺:
- Serum (pH ~7.4)
- 20% dihydrogen phosphate (H₂PO₄⁻)
- 80%
monohydrogen phosphate (HPO₄⁻⁻)
- Urine (pH ~6.8)
- 50% dihydrogen phosphate (20%
 50%)
50%) - 50%
monohydrogen phosphate (80% 50%)
50%)
- 50% dihydrogen phosphate (20%
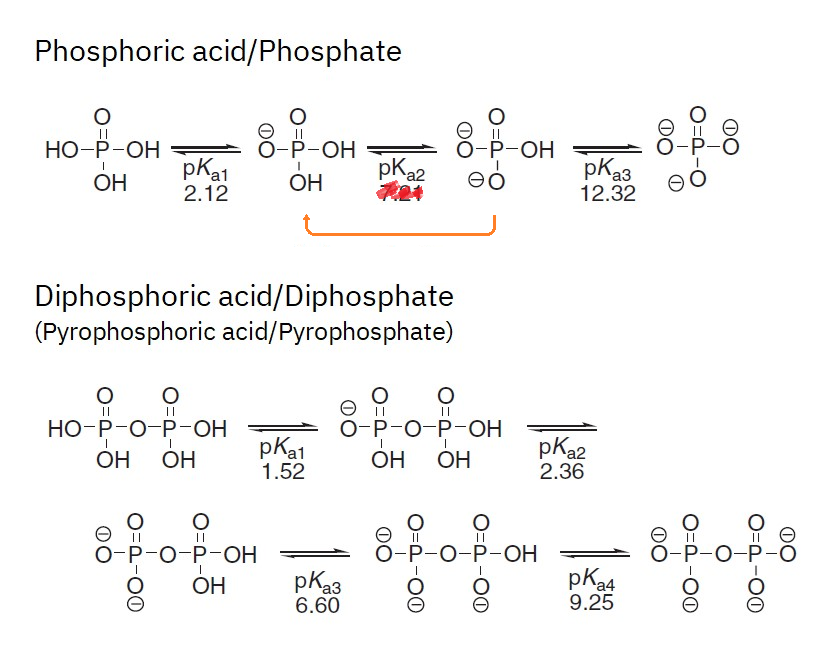
(10.1002/9780470048672.wecb641)So, for a given quantity of phosphate, the species redistribution represents additional protons being accepted by phosphates and taken out.
Depending on the charge, an ion can pair with more or less counterions to maintain neutrality. The Equivalent unit (Eq) accounts for these charge differences.
Predictable values would be:
- 100% as dihydrogen phosphate (H₂PO₄⁻): mEq = 1
- 100% as hydrogen phosphate (HPO₄⁻⁻): mEq = 2
For example, if sodium ions were to pair with them, one ion would be needed for dihydrogen phosphate (NaH₂PO₄) and two for hydrogen phosphate (Na₂HPO₄).
However, it's always a combination of these species in the body, that calls for an approximate mEq value somewhere between 1-2:
-
Serum
- 20% (dihydrogen phosphate) * 1 mEq
- 80% (monohydrogen phosphate) * 2 mEq
- mEq = 0.2 + 1.6 = 1.8
-
Urine
- 50% (dihydrogen phosphate) * 1 mEq
- 50% (monohydrogen phosphate) * 2 mEq
- mEq = 0.5 + 1.0 = 1.5
So, when you spot this unit applied to phosphate in medicine, it should be an approximation based on mixed charges.
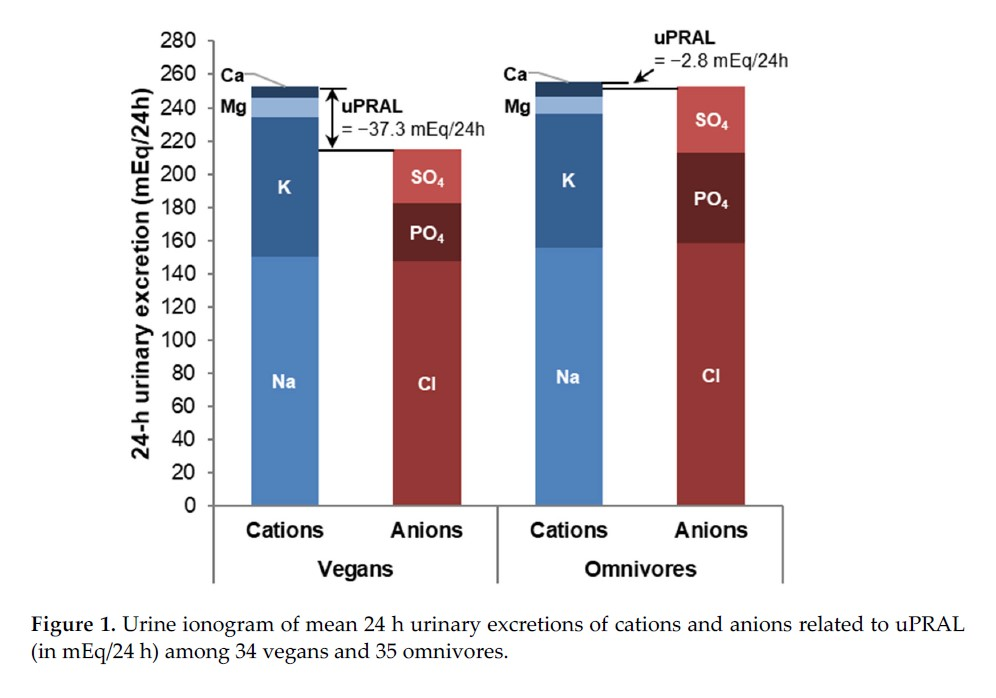
(10.3390/nu14214468)For a victim that excretes 20 mmol of phosphate in a day, we would have in total:
Serum:
- 4 mmol H₂PO₄⁻ (20%)
- 16 mmol HPO₄⁻⁻ (80%)
Urine:
- 10 mmol H₂PO₄⁻ (50%)
- 10 mmol HPO₄⁻⁻ (50%)
The change:
- 10 − 4 mmol = 6 mmol H⁺/day
Therefore, out of 20 mmol eliminated in a day, 6 mmol would represent acid excretion and the rest of phosphates are excreted without accepting more protons because they were already loaded.
You can search for calculators that 'convert pH to molar concentrations' for precise values, but at the extreme condition of urine pH of 4.4, the proton concentration would be something like 0.04 mmol H⁺/L (0.00004 mol H⁺/L).
Protons elimination:
- Through phosphate: 6 mmol H⁺/day
- Free: 0.06 mmol H⁺/day (from: 0.04 mmol H⁺/L urine × 1.5 L urine/day)
But there's a problem..
The original form of phosphorus
Most of the attention is on renal metabolism and excretion, whereas the ingested form is usually neglected.
When we know how a compound entered and left the body, it's possible to have a better idea if there was overall gain or loss of protons.
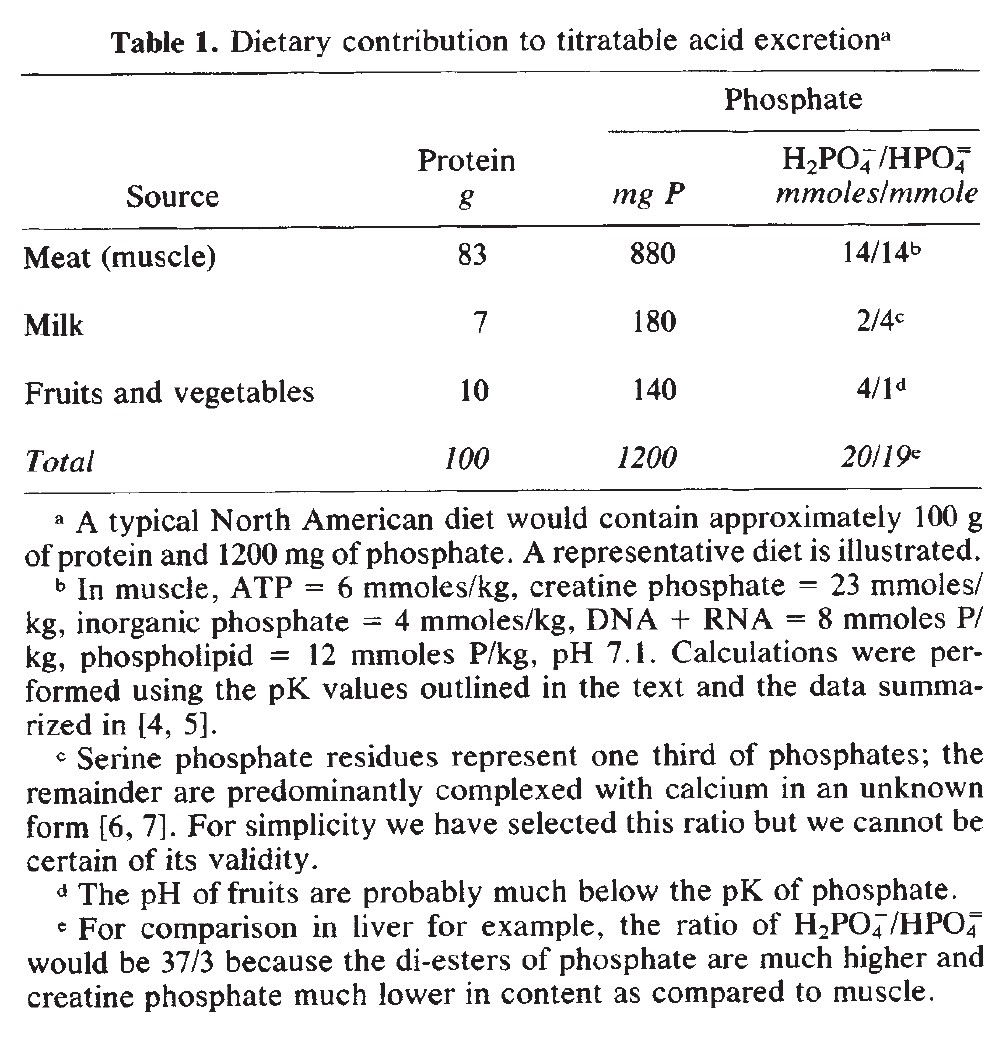
(10.1038/ki.1983.217)If the ingested phosphate is derived from acidic foods or those that yield dihydrogen phosphate after phosphocompounds are broken down, minimal net loss of protons from the body occurs in the grand picture, as the phosphates in question wouldn't accept more hydrogens for having the respective sites are already occupied. That is, unless the urine is acidified (allowing phosphates to accept more protons). However, beyond the relevant buffering range of phosphate, the body must resort to other buffers or mobilize stored phosphate.
(10.1007/978-1-4614-3770-3_7)In extreme situations, ketones (mainly hydroxybutyrate) become significant urinary buffers as well.
For an animal in normal conditions, whose diet after digestion yielded similar phosphate species as that of urine, the net effect on protons would be neutral. It may seem an obvious statement, but if we only compare the compounds in urine to those in serum, it could mislead, making it seem to represent acid excretion.
Bone as a buffer
The hydrogens are released as protons to prepare the phosphates to be incorporated in (hydroxy)apatite. The protons released result in acidification.
Two representations of hydroxyapatite:
- 3[Ca₃(PO₄)₂]⋅Ca(OH)₂ (calcium phosphate 3:1 calcium hydroxide)
- Ca₁₀(PO₄)₆(OH)₂
10 parts of calcium for 6 parts of phosphorus is the synthetic molar ratio, which can be simplified:
- Ca 10:6 P
- Ca 1.67:1 P
In bone formation, phosphorus (as phosphate) is derived from circulation for deposition, where have the phosphate species in the expected proportions because of pH (and other factors):
Serum
- 20% dihydrogen phosphate × 6 = 1.2 dihydrogen phosphate
- 80% monohydrogen phosphate × 6 = 4.8 monohydrogen phosphate
The reaction can be represented as follows:
- 10 [Ca²⁺] + 1.2 [H₂PO₄⁻] + 4.8 [HPO₄²⁻] + 2 [H₂O] ⇄ [Ca₁₀(PO₄)₆(OH)₂] + 9.2 [H⁺]
Protons Source 2 × 1.2 H⁺ H₂PO₄⁻ 4.8 H⁺ HPO₄²⁻ 2 H⁺ 2H₂O: 2H⁺ + 2OH⁻ 9.2 H⁺ ← Total released per hydroxyapatite formed The result is acidification in bone formation or alkalinization in bone dissolution, when the reaction above is reversed (right to left), consuming an equivalent amount of protons.
Therefore, bone synthesis and break down can help to regulate acidity (as drugs) when other systems are not enough. Unlike dietary phosphate that's preloaded with hydrogen atoms, bone phosphate is not, and can take up a substantial amount as protons for excretion. However, note that it's a short-term effect, and we return to the same issue: phosphate was once derived from the diet with the respective release of protons.
Immobilization can lead to a marked loss of bone, independent of diet, in particular during the initial period. The amount of calcium lost may double.
- Serum (pH ~7.4)
-
@Amazoniac Thank you for the comprehensive information!!
And Hail Satan! i.e. Hail Phosphorus I meant...

-
Thank you for collecting all of the detailed information about phosphorus.
@Amazoniac said in Phosphorus, the toxin:
When we connect them, the message is clear.

What authorities are trying to accomplish by recommending its consumption?
This is yet another instance of the health authorities making recommendations that undermine our health. They have gotten the facts wrong so many times in a row that it appears as if they are puposefully being indifferent to the consequences.
-
Peatbot: Paying attention to the calcium to phosphorus ratio is important because it has significant implications for your overall health, particularly in terms of bone health, metabolic function, and the prevention of certain chronic conditions. Here are some key reasons:
-
Bone Health: Calcium and phosphorus are critical components of bone. An imbalance, particularly an excess of phosphorus relative to calcium, can lead to the mobilization of calcium from bones, weakening them and increasing the risk of fractures and osteoporosis.
-
Metabolic Function: Phosphorus is involved in energy metabolism, particularly in the formation of ATP (adenosine triphosphate), which cells use for energy. However, excessive phosphorus can disrupt this balance and lead to metabolic inefficiencies.
-
Parathyroid Hormone Regulation: High phosphorus levels can trigger the release of parathyroid hormone (PTH), which works to balance calcium and phosphorus levels in the blood. Chronic elevation of PTH can lead to bone resorption, where calcium is taken from the bones to balance the high phosphorus levels, leading to weakened bones.
-
Inflammation and Degeneration: An excess of phosphorus can contribute to inflammation and tissue degeneration. It can lead to the formation of calcium phosphate crystals in soft tissues, which are associated with chronic inflammation and degenerative diseases.
-
Calcium Deposition: High phosphorus levels can cause calcium to be deposited in soft tissues, such as arteries, rather than in bones. This can contribute to conditions like arteriosclerosis and other cardiovascular diseases.
-
Nutrient Interactions: The balance of calcium and phosphorus affects the absorption and utilization of other minerals, such as magnesium and sodium. An imbalance can disrupt these interactions and lead to deficiencies or excesses that affect overall health.
In summary, maintaining a proper calcium to phosphorus ratio is crucial for ensuring that calcium is used effectively in the body, supporting bone health, preventing chronic diseases, and maintaining overall metabolic balance.
-
-
@Amazoniac
thanks
having some sugar / fructose with a high phosphorus meal can counter absorption well too
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2430509/
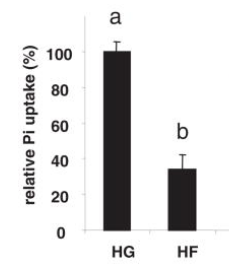
(maybe too much at once for liver to deal with effectively in this study but shows big effect)- If shown to lower serum Pi, consumption of fructose can also have dramatic implications for Pi metabolism and hyperphosphatemia in renal failure because even slight reductions in serum Pi concentrations result in marked decreases in mortality risk
So calcium & or fructose with phosphorous meals to balance absorption if on high phosphorus intake
(or on the other side to avoid these simultaneously with phosphate if actively trying to build bone
ray mentioned in his article low phosphate intake can also be a problem because it has the same effects as high phosphate from boosting absorption & increasing transporter for more cellular uptake, tho maybe using fructose/calcium regularly would keep the transporter inhibited)(1,25 vit D also increases phosphate absorption) https://pubmed.ncbi.nlm.nih.gov/12032488/
reposting https://raypeat.com/articles/articles/phosphate-activation-aging.shtml
- In the kidneys, phosphate promotes calcification (Bois and Selye, 1956), and osteopontin, by its activation of inflammatory T-cells, is involved in the development of glomerulonephritis, as well as in inflammatory skin reactions (Yu, et al., 1998).
High dietary phosphate increases serum osteopontin, as well as serum phosphate and parathyroid hormone, and increases the formation of tumors in skin (Camalier, et al., 2010). Besides the activation of cells and cell systems, phosphate (like other ions with a high ratio of charge to size, including citrate) can activate viruses (Yamanaka, et al., 1995; Gouvea, et al., 2006).
Aromatase, the enzyme that synthesizes estrogen, is an enzyme that's sensitive to the concentration of phosphate (Bellino and Holben, 1989). - A diet that provides enough calcium to limit activity of the parathyroid glands, and that is low in phosphate and polyunsaturated fats, with sugar rather than starch as the main carbohydrate, possibly supplemented by niacinamide and aspirin,
should help to avoid some of the degenerative processes associated with high phosphate: fatigue, heart failure, movement discoordination, hypogonadism, infertility, vascular calcification, emphysema, cancer, osteoporosis, and atrophy of skin, skeletal muscle, intestine, thymus, and spleen (Ohnishi and Razzaque, 2010; Shiraki-Iida, et al., 2000; Kuro-o, et al., 1997; Osuka and Razzaque, 2012). - An extreme reduction of phosphate in the diet wouldn't be appropriate, however, because a phosphate deficiency stimulates cells to increase the phosphate transporter, increasing the cellular uptake of phosphate, with an effect similar to the dietary excess of phosphate, i.e., promotion of lung cancer (Xu, et al., 2010) https://pubmed.ncbi.nlm.nih.gov/20432174/ {lowering to 20% of control diet}. The optimum dietary amount of phosphate, and its balance with other minerals, hasn't been determined.
-
“ So calcium & or fructose with phosphorous meals to balance absorption if on high phosphorus intake”
So what would be some examples of phosphorus dominant meals?
-
@Butter-Girl meals high in meat , seafood, some wholegrains if eating for the fiber & nutrients e.g spelt (ray said some of his teeth decayed from a time where he was eating a lot of wheat germ for the nutrients without calcium)