Chris Masterjohn Substack
-
He made a post on carbs producing oxalates but it's paywalled
oxalate levels within the normal range inhibits the Krebs cycle by about 50%
if anyone here is subscribed could you give a summary
here's the link:
https://chrismasterjohnphd.substack.com/p/are-sugar-and-xylitol-behind-your -
@Peter_ bump
-
@Peter_ said in Chris Masterjohn Substack:
oxalate levels within the normal range inhibits the Krebs cycle by about 50%
Main ideas
I haven’t the whole PDF of CM but here is a post I made about oxalate (In French, translator needed; but the links are mostly in English if you want to go deeper into the mechanism).
Negative impact of oxalate on metabolism: Biochemical havoc!
Impact négatif de l’oxalate sur le métabolisme : Chaos biochimique !
https://mirzoune-ciboulette.forumactif.org/t1959-impact-negatif-de-loxalate-sur-le-metabolisme-chaos-biochimique#28275
=> Shortly said: We can manage a small amount of oxalates.When we overload the liver, we need more Sulphur to neutralize oxalate. We lost then methylation capacity.
Some useful nutrients are no longer available, like B6. Problems are going to go upwards: lack of enzymes, dysregulation of neurotransmitters and less detox…
Excerpt 1:
Most people are not aware of the problem. The oxalates generate a large biochemical chaos in the body (3), more than any other component (if we except mercury). Problems related to sulfur (and by ricochet to histamine and salicylates) can be caused by problematic metabolism, which can no longer treat excess of foods rich in oxalates. It is the whole metabolism (the liver via different systems related to sulfate, methylation) and deprivation of nutrients useful for intestinal bacteria, which are altered. For example, pyroxidine (B6) is exhausted in this process of neutralization of oxalates. We need B6 in the process which consists in exchanging an oxalate molecule with a sulphate molecule. The B6 is necessary for the operation of around 150 enzymes linked to around 60 genes which regulate neurotransmitters and detoxification of the liver as well as general metabolism. (4)
Excerpt 2
Biochemical problems associated with weak phenotype in sulfate and rich in oxalate- Slowed growth
- Slow metabolism
- Modified behavior
- Decrease in insulin function
- High total LDL and cholesterol
- Increase in liver stress and foie gras
- Altered detox and SULT genes upregulated
- Low cortisol, deficit in DHEA and adrenal hormones
- Increase in colitis and inflammatory intestine diseases
- Decrease in mucus production in the intestine
- Increase in intestinal permeability (Leaky Gut)
- Sensitivity to aggressive intestinal bacteria
- Reduction of the expression of metallothione and detoxification of heavy metals
- Increase in the size and vascularization of tumors
- Excess serotonin in the blood and decrease in serotonin in the brain
- Autism linked to the waste of sulfate
Parenthesis
Note that this could be a mechanism for the defense of certain bacterial strains, including Candida Albicans, in the presence of an excess of Oxalate. (5) We need sulfur to evacuate the excess of Oxalate. As the oxalates are toxic to the microbiota, some strains are overexcited (Quorum Sensing) (6-7) and proliferate…
End of the parenthesis. See also below, the reflections of researcher Susan Owens, to meditate on the oxalates candidiasis, SIBO and other dysbiosis. (5)
-
@Peter_ said in Chris Masterjohn Substack:
oxalate levels within the normal range inhibits the Krebs cycle by about 50%
Low oxidation of carbs when B6 is depleted
We know that excess of oxalates exhausts pyridoxine (B6).
B6 is a cofactor for one of enzymes degrading oxalate in the body. Excess oxalate is going then to deplete B6. As well when there is latent inflammation B6 is burnt (B6 acts as antioxidant too).
Pyridoxine is a coenzyme: B6 assists in amino-acid synthesis, glycogneolysis, neurotransmitter and hemoglobin synthesis.
Vitamin B6 can reduce postprandial blood glucose levels following sucrose and starch ingestion.
doi: 10.3390/ijms21103669 -
More search has given the following results.
Oxalates also interfere with the Krebs cycle's glucose metabolism and can inhibit absorption of essential minerals necessary for optimum health.
Oxalate, or oxalic acid, is a metabolic breakdown product in the Krebs Cycle.
Oxalate apparently would act by inhibiting pyruvate carboxylase.
=> Excess oxalate could deplete pyruvate carboxylase.
Pyruvate carboxylase (PC) catalyzes the conversion of pyruvate to oxaloacetate
But for CM it has never been demonstrated that pyruvate carboxylase can decarboxylate oxalate.Inhibitors of Pyruvate Carboxylase
See & Oxalate
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3238542/
Oxalate, an inhibitor of both lactate dehydrogenase [72] and transcarboxylase [73], was also found to be an effective inhibitor for PC from a variety of sources. (…)
PC = pyruvate carboxylase
Similar to oxamate, oxalate also inhibits gluconeogenesis in isolated mitochondria and hepatocytes. Dennis et al. [76] determined that oxalate had no inhibitory effect on pyruvate transportation into the mitochondria; therefore the inhibition of glucose production was presumably due to the direct inhibition of PC by oxalate. -
I am curious about the role of calcium in oxalate metabolism.
I am under the impression that calcium aids in the excretion of oxalates making dairy an ideal pairing with oxalate-rich foods (cheese + potatoes).
-
@AstralPMP said in Chris Masterjohn Substack:
I am under the impression that calcium aids in the excretion of oxalates making dairy an ideal pairing with oxalate-rich foods (cheese + potatoes).
Reply
Yes, I can confirm. But only during digestion.
But only if the amount brought is optimal. Not much but enough to form a amalgam (a kind of soap) evacuated through the stools, and partly through urine if dissolved.
Ca is optimal between 800 and 1200 mg daily.
Source : DOI: https://doi.org/10.1097/01 Gerd E. von Unruh, Susanne Voss et al. JASN June 2004.
https://jasn.asnjournals.org/content/15/6/1567
ABSTRACT.
2 to 20% of ingested oxalate is absorbed in the gastrointestinal tract of healthy humans with a daily 800 mg calcium intake. Calcium is the most potent modifier of the oxalate absorption. Oxalate absorption depends linearly on the calcium intake.
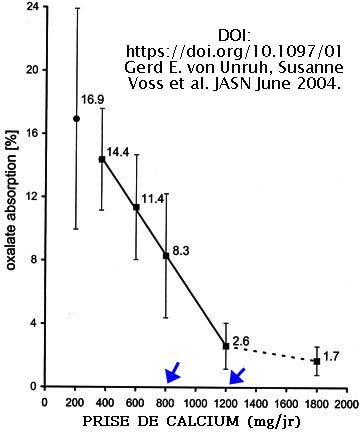
-
useful info for prevention of lithiasis (calcium stones)
Citrates to prevent calcium and uric acid stones
By Fredric Coe, MD
Reduced risk of crystal formation for oxalate and phosphate stones
Up to this point most of MD have considered only increase of urine volume as a means of stone prevention. Drink at least 2 liters source water to help eliminate stones.
Why CitratesCitrate is an inhibitor of crystallization both because it is calcium binding and because it directly affects calcium crystal growth.
Mechanisms
Supersaturation with respect to the calcium stones depends upon urine concentrations of calcium, oxalate, phosphate, and citrate, and, in the case of calcium phosphate stones, or uric acid stones, urine pH. Giving citrate salts can reduce urine calcium excretion and increase urine citrate. Urine citrate binds urine calcium in a soluble citrate complex, which reduced calcium salt supersaturations. Citrate inhibits crystal formation, growth and aggregation. The alkaline citrate salts can raise urine pH.
https://kidneystones.uchicago.edu/citrate-to-prevent-stones/
Note: I use calcium citrate powder in a shake (1.3 gr).
Remember: Only 30 % of Ca is assimilated from milk and cheese. 66 % from broccoli. -
@LucH Very interesting. It would seem then that the inhibiting effect of calcium on oxalate absorption is limited to interaction within the gut, meaning one should eat calcium at the same time or immediately prior to eating oxalate-rich food. If you wait too long to eat your cheese after your potatoes, you will miss out on the effect.
-
@AstralPMP said in Chris Masterjohn Substack:
t would seem then that the inhibiting effect of calcium on oxalate absorption is limited to interaction within the gut, meaning one should eat calcium at the same time or immediately prior to eating oxalate-rich food. If you wait too long to eat your cheese after your potatoes, you will miss out on the effect.
Yes, but you have some time before it gets stuck. +/ 25 'in the stomach before emptying the food bowl (most likely to form a kind of soap). Then in the colon, with the chyme (food bowl).
I did not check if it happens like this. Deduction.
I will see if I find more details.
I would not limit myself to calcium. Calcium and citrate, not necessarily at the same time. Citrates when you want (dissolution). -
@LucH said in Chris Masterjohn Substack:
I will see if I find more details.
=> the only senseful explanation I've read:
The time before it reaches the kidney.
This explanation is particularly appropriate for citrate.
A little amount of vitamin C is useful too. An orange or mandarin is enough. Not during the crisis because the residues leave little oxalate.
For milk or cheese, I won't take it later than 30' after the meal,
Of course if you don't reach 850 mg Ca, it would be useful at any moment to avoid the action of PTH. -
@LucH Does taking citrate or vitamin C block absorption of oxalate in the gut similar to calcium? Or is it that they change the PH in the kidneys which breaks down oxalate crystals?
-
*) Assimilating vitamin C supplement is leaving oxalate
As little 250 – 500 mg L-ascorbic has been tested and is counter-productive.
Excerpt:
Moreover, vitamin C supplementation at daily dosages of both 250–499 mg and 1000–1499 mg was associated with an 11–14% increased risk of kidney stones. It is remarkable that it occurs in males but not in females [20,23]. Differences by gender are present also for Vitamin B6 intake.
Vitamin B6 is a cofactor of oxalate metabolism.
See figure 2 for optimal substance claiming to treat kidney stones.
doi: 10.3390/nu15040877 2023
"Kidney Stone Prevention: Is There a Role for Complementary and Alternative Medicine?"
Efficacy rate:
High efficacity (40%) for potassium and magnesium citrate and plant extract from phyllantus niruri (Echinacea). Phyllanthus emblica and Phyllanthus niruri co-exist. Verify with the Latin name. Mind the use of this plant since it has an impact on platelet aggregation (inhibition).
Personal adviceI won’t take vitamin C supplement during a crisis.
Avoid vitamin C supplement is mostly adviced. But I don’t agree: I manage well with a tiny amount when modulating the whole thing: I take a very / very small pinch (0.12 gr) with 100 ml water. I drink little mineralized water, spring water with. Half-life of vitamin C is short. Twice a day would be optimal in case of inflammation, if you manage well.
To balance my recommended intake of potassium (4500 mg RDA), I take some potassium citrate (1 scope 1.3 gr) and for magnesium (420 mg RDA) I take one scope magnesium bisglycinate (2.5 gr). I use cronometer.com as monitoring software.
Of course, we need enough Ca (800 – 1200 mg) from food. Preferably from food. Never take a calcium supplement above 200-250 mg. Calcium citrate powder is advised.
One scope 1.3 gr calcium citrate brings +/ 1/3 Ca element (400 mg). Taken in 2 doses.
Mind capsule saying 900 mg. It only contains 1/3 Ca.Microbiote
After taking an antibiotic, some remaining strains could manage oxalate (sensing chorum), Unbalanced microbiote. Must be reloaded.High food oxalate
High-oxalate foods. Many plants contain oxalate, so it’s hard to avoid it entirely. But some foods have much more than others. Try to limit:
• Spinach
• Rhubarb
• Almonds and cashews
• Miso soup
• Grits
• Baked potatoes with skin
• Beets
• Cocoa powder
• Okra
• Bran cereals
• French fries
• Raspberries
• Stevia sweeteners
• Sweet potatoes
• 1 Medjol Date = 24 mg
• + tea / coffee.
https://ucikidneystonecenter.com/wp-content/uploads/2020/06/Oxalate-Content-of-Foods.pdf
If you eat or drink calcium-rich foods at the same time, they can help your body handle oxalate without turning it into a kidney stone. So pair your spinach salad with low-fat cheese. Or mix nuts or berries into yogurt. Drinking milk does not cause kidney stones. Mind cheese rich in phosphorus, like Gouda cheese. Optimal ratio of P / Ca is 1 / 2.2 (Ray PEAT).
I often eat Greek yoghurt (10 % fat) or white cheese (Campina 8.8 gr fat) mixed with fresh fruit. Strawberries (4 g / 100 g is rather high in oxalate) cut into pieces or blueberries or any other season fruit if it doesn’t contain many seeds.As long you don’t overload (maxi 50 mg), you can manage oxalate residue. 100 - 200 mg oxalate is considered as maximum amount being able to be treated, if not reached every day.
AstralPMP asked:
"Does taking citrate or vitamin C block absorption of oxalate in the gut similar to calcium?"
=> Citrate doesn’t block absorption. Citrate contributes to dilution of oxalate.
Citrate reduces CaOx crystal aggregation and aids other crystallization inhibitors. Alkaline urine reduces urine calcium excretion.
Vocabulary explanation:- Potassium citrate or magnesium citrate makes oxalate more fluid, preventing from aggregation and then not allowing crystallization (no stone). The action is not guaranteed (40 % efficacy) (= 40% less stones by weak persons).
- If you balance well acid-basic minerals, you won’t excrete much Ca in urine.
Note: Taking high amount of sodium requires Ca from bones to balance blood Ph. Optimal ratio Na / K would be 1 / 2. I don’t follow RDA recommendation for sodium (below 2.5 gr sodium = 6 gr salt) as it’s obsolete. As long as you get enough potassium and you follow your sensation it’s going to be fine. But if you eat manufactured food, you’ll probably need an adaptation period to reset metabolic sensors (body signals).
=>Optimal amount of daily Ca is between 800 – 1200 mg, preferably from food.
Ca amalgams with oxalate in the stomach (mainly in the stomach) and forms a soap, evacuated in stools. What is not amalgamated is got rid of through the kidneys, in urine.
Note: When excess fat is not well assimilated.
When fat is not absorbed the right way, the fat binds to calcium and leaves oxalate behind.
https://www.kidney.org/kidney-topics/calcium-oxalate-stones -
@LucH said in Chris Masterjohn Substack:
*) Assimilating vitamin C supplement is leaving oxalate
As little 250 – 500 mg L-ascorbic has been tested and is counter-productive.
Excerpt:
Moreover, vitamin C supplementation at daily dosages of both 250–499 mg and 1000–1499 mg was associated with an 11–14% increased risk of kidney stones. It is remarkable that it occurs in males but not in females [20,23]. Differences by gender are present also for Vitamin B6 intake.
Vitamin B6 is a cofactor of oxalate metabolism.
See figure 2 for optimal substance claiming to treat kidney stones.
doi: 10.3390/nu15040877 2023
"Kidney Stone Prevention: Is There a Role for Complementary and Alternative Medicine?"
Efficacy rate:
High efficacity (40%) for potassium and magnesium citrate and plant extract from phyllantus niruri (Echinacea). Phyllanthus emblica and Phyllanthus niruri co-exist. Verify with the Latin name. Mind the use of this plant since it has an impact on platelet aggregation (inhibition).
Personal adviceI won’t take vitamin C supplement during a crisis.
Avoid vitamin C supplement is mostly adviced. But I don’t agree: I manage well with a tiny amount when modulating the whole thing: I take a very / very small pinch (0.12 gr) with 100 ml water. I drink little mineralized water, spring water with. Half-life of vitamin C is short. Twice a day would be optimal in case of inflammation, if you manage well.
To balance my recommended intake of potassium (4500 mg RDA), I take some potassium citrate (1 scope 1.3 gr) and for magnesium (420 mg RDA) I take one scope magnesium bisglycinate (2.5 gr). I use cronometer.com as monitoring software.
Of course, we need enough Ca (800 – 1200 mg) from food. Preferably from food. Never take a calcium supplement above 200-250 mg. Calcium citrate powder is advised.
One scope 1.3 gr calcium citrate brings +/ 1/3 Ca element (400 mg). Taken in 2 doses.
Mind capsule saying 900 mg. It only contains 1/3 Ca.Microbiote
After taking an antibiotic, some remaining strains could manage oxalate (sensing chorum), Unbalanced microbiote. Must be reloaded.High food oxalate
High-oxalate foods. Many plants contain oxalate, so it’s hard to avoid it entirely. But some foods have much more than others. Try to limit:
• Spinach
• Rhubarb
• Almonds and cashews
• Miso soup
• Grits
• Baked potatoes with skin
• Beets
• Cocoa powder
• Okra
• Bran cereals
• French fries
• Raspberries
• Stevia sweeteners
• Sweet potatoes
• 1 Medjol Date = 24 mg
• + tea / coffee.
https://ucikidneystonecenter.com/wp-content/uploads/2020/06/Oxalate-Content-of-Foods.pdf
If you eat or drink calcium-rich foods at the same time, they can help your body handle oxalate without turning it into a kidney stone. So pair your spinach salad with low-fat cheese. Or mix nuts or berries into yogurt. Drinking milk does not cause kidney stones. Mind cheese rich in phosphorus, like Gouda cheese. Optimal ratio of P / Ca is 1 / 2.2 (Ray PEAT).
I often eat Greek yoghurt (10 % fat) or white cheese (Campina 8.8 gr fat) mixed with fresh fruit. Strawberries (4 g / 100 g is rather high in oxalate) cut into pieces or blueberries or any other season fruit if it doesn’t contain many seeds.As long you don’t overload (maxi 50 mg), you can manage oxalate residue. 100 - 200 mg oxalate is considered as maximum amount being able to be treated, if not reached every day.
AstralPMP asked:
"Does taking citrate or vitamin C block absorption of oxalate in the gut similar to calcium?"
=> Citrate doesn’t block absorption. Citrate contributes to dilution of oxalate.
Citrate reduces CaOx crystal aggregation and aids other crystallization inhibitors. Alkaline urine reduces urine calcium excretion.
Vocabulary explanation:- Potassium citrate or magnesium citrate makes oxalate more fluid, preventing from aggregation and then not allowing crystallization (no stone). The action is not guaranteed (40 % efficacy) (= 40% less stones by weak persons).
- If you balance well acid-basic minerals, you won’t excrete much Ca in urine.
Note: Taking high amount of sodium requires Ca from bones to balance blood Ph. Optimal ratio Na / K would be 1 / 2. I don’t follow RDA recommendation for sodium (below 2.5 gr sodium = 6 gr salt) as it’s obsolete. As long as you get enough potassium and you follow your sensation it’s going to be fine. But if you eat manufactured food, you’ll probably need an adaptation period to reset metabolic sensors (body signals).
=>Optimal amount of daily Ca is between 800 – 1200 mg, preferably from food.
Ca amalgams with oxalate in the stomach (mainly in the stomach) and forms a soap, evacuated in stools. What is not amalgamated is got rid of through the kidneys, in urine.
Note: When excess fat is not well assimilated.
When fat is not absorbed the right way, the fat binds to calcium and leaves oxalate behind.
https://www.kidney.org/kidney-topics/calcium-oxalate-stonesDo you need to take potassium citrate or can you just get enough of it from fruits like bananas?
-
@Butter-Girl said in Chris Masterjohn Substack:
Do you need to take potassium citrate or can you just get enough of it from fruits like bananas?
2 things:
- Potassium
I hardly reach most of the RDA (420 for M, 350 for W) with two fruits at breakfast, one orange and a peach at midday and half a melon at evening meal. => 3/4 reached.
The banana is interesting but no more than an orange, except it's more alcalin. However, orange contain some oxalate too. Not the right choice if you are borderline with histamine (both fruits).
If you add 2 medium pealed potato (not sweat potato), it will be OK, but you need to add some fat (butter + egg yolk) for dealing with glycemia, and vegetables. Broccoli would be fine. Not spinach, very rich in oxalate (skyrocketed: 600 mg)
Note: You go to stools 2x/d when eating starch or you'll get problems soon or later with microbiote (dysbiosis), if repeated. - It's citrate who is required to solubilize oxalate.
120 ml lemon juice could do the job, mixed with 500 ml water. But you won't be able to drink this drink every day (sensibility of stomach: mucin not thick enough).
Conclusion: I take Now Foods Potassium citrate. Mind the humidity to avoid amalgam of the powder. I put a safe bandelette in the container.
- Potassium
-
Thank you for your detailed response.
-
@LucH said in Chris Masterjohn Substack:
Potassium
I hardly reach most of the RDA (420 for M, 350 for W)The RDA for potassium is much higher.
-
@LucH said in Chris Masterjohn Substack:
It's citrate who is required to solubilize oxalate.
120 ml lemon juice could do the job, mixed with 500 ml water.The lemon juice has citric acid. Need to mix with baking soda to produce sodium citrate. I mix 16ml lemon juice with 1g baking soda to make sodium citrate. It's sodium though, but I don't mind as it's the citrate I want.
I had a bad experience with using industrially made potassium citrate. It is made by black molds. See haidut's thread on RPF.
Soon after that thread was started, I developed a really bad infection and when I recovered, I went back to my logs and realized I had taken potassium citrate for increasing by blood flow (via increasing zeta-value) and I had also unintentionally taken it while I was using tetracycline to treat infection causing my high BP.
I connected the dots and realized that the trace of aspergillus from the potassium citrate made the cell wall deficient bacteria (morphed from using tetracycline) morphed further into a more virulent fungal parasites.
Since then, I have been very cautious about using industrially made potassium citrate.
-
@LucH Thanks for the great info!