Metformin reduces gluconeogenesis that is typical of "fight or flight" stress.
-
Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase
https://www.nature.com/articles/nature13270The ‘Jekyll and Hyde’ of Gluconeogenesis: Early Life Adversity, Later Life Stress, and Metabolic Disturbances
The physiological response to a psychological stressor broadly impacts energy metabolism. Inversely, changes in energy availability affect the physiological response to the stressor in terms of hypothalamus, pituitary adrenal axis (HPA), and sympathetic nervous system activation. Glucocorticoids, the endpoint of the HPA axis, are critical checkpoints in endocrine control of energy homeostasis and have been linked to metabolic diseases including obesity, insulin resistance, and type 2 diabetes. Glucocorticoids, through the glucocorticoid receptor, activate transcription of genes associated with glucose and lipid regulatory pathways and thereby control both physiological and pathophysiological systemic energy homeostasis. Here, we summarize the current knowledge of glucocorticoid functions in energy metabolism and systemic metabolic dysfunction, particularly focusing on glucose and lipid metabolism. There are elements in the external environment that induce lifelong changes in the HPA axis stress response and glucocorticoid levels, and the most prominent are early life adversity, or exposure to traumatic stress. We hypothesise that when the HPA axis is so disturbed after early life adversity, it will fundamentally alter hepatic gluconeogenesis, inducing hyperglycaemia, and hence crystalise the significant lifelong risk of developing either the metabolic syndrome, or type 2 diabetes. This gives a “Jekyll and Hyde” role to gluconeogenesis, providing the necessary energy in situations of acute stress, but driving towards pathophysiological consequences when the HPA axis has been altered.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8037741/Association between Metformin Treatment and Improved Symptoms of Post-Traumatic Stress Disorder
https://diabetesjournals.org/diabetes/article/69/Supplement_1/174-LB/56254Metformin Use Associated with Decreased PTSD Symptoms in Diabetic Veterans
https://www.hcplive.com/view/metformin-associated-with-decreased-ptsd-symptoms-in-diabetic-veteransMetformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation
https://pubmed.ncbi.nlm.nih.gov/31521867/To counteract possible lactic acidosis induced by metformin, simply add a teaspoon of calcium carbonate to your favourite beverage:
Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin
Abstract
Objective: Of patients who are prescribed metformin, 10-30% have evidence of reduced vitamin B12 absorption. B12-intrinsic factor complex uptake by ileal cell surface receptors is known to be a process dependent on calcium availability Metformin affects calcium-dependent membrane action. The objective of this study was to determine the magnitude and mechanism of the reduction in serum vitamin B12 after metformin administration.Research design and methods: A comparative study design was employed using 2 groups (metformin and control). A total of 21 patients with type 2 diabetes received sulfonylurea therapy; 14 of these 21 patients were switched to metformin. Monthly serum total vitamin B12 measurements and holotranscobalamin (holoTCII) (B12-TCII) were performed. After 3 months of metformin therapy, oral calcium supplementation was administered.
Results: Serial serum vitamin B12 determinations revealed a similar decline in vitamin B12 and holoTCII. Oral calcium supplementation reversed the metformin-induced serum holoTCII depression.
Conclusions: Patients receiving metformin have diminished B12 absorption and low serum total vitamin B12 and TCII-B12 levels because of a calcium-dependent ileal membrane antagonism, an effect reversed with supplemental calcium.
https://pubmed.ncbi.nlm.nih.gov/10977010/ -
Mind-body practices lower blood sugar levels in people with type 2 diabetes
All practices achieve significant reductions in blood sugar levelsThe team analyzed data from randomized controlled trials conducted across the globe between 1993 and 2022. They found 28 trials in which people with type 2 diabetes began a mind-body practice in addition to receiving medication and compared their results with people who only received medication to reduce their blood sugar levels.
This study, the first to analyze a range of mind-body practices including meditation, qigong, yoga and mindfulness-based stress reduction and their effect on blood glucose levels, revealed that all mind-body practices led to significant reductions in blood sugar levels.
Taken as a whole, the mind-body practices averaged a .84% reduction in hemoglobin A1c, a measure of the average blood glucose level for the past 3 months. Yoga, the most-studied modality, provided the largest benefit, about a 1% reduction in hemoglobin A1c. The authors noted that a 1% reduction is particularly notable because metformin, the most prescribed diabetes drug, reduces hemoglobin A1c in people with type 2 diabetes by 1.1% on average.
“What is important about this study is that the effect is very strong and that it is on top of the standard of care,” said Richard M. Watanabe, PhD, professor of population and public health sciences and physiology and neuroscience at the Keck School of Medicine, noting that the research revealed that mind-body practices helped participants achieve reductions in blood glucose levels on top of the reductions they were getting from medication.
https://keck.usc.edu/news/mind-body-practices-lower-blood-sugar-levels-in-people-with-type-2-diabetes/Effect of 6 Months of Meditation on Blood Sugar, Glycosylated Hemoglobin, and Insulin Levels in Patients of Coronary Artery Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934947/Effects of Buddhist walking meditation on glycemic control and vascular function in patients with type 2 diabetes
Results
After 12 weeks, maximal oxygen consumption increased and fasting blood glucose level decreased significantly in both groups (p < 0.05). Significant decrease in HbA1c and both systolic and diastolic blood pressure were observed only in the WM group. Flow-mediated dilatation increased significantly (p < 0.05) in both exercise groups but arterial stiffness was improved only in the WM group. Blood cortisol level was reduced (p < 0.05) only in the WM group.Conclusion
Buddhist walking meditation exercise produced a multitude of favorable effects, often superior to traditional walking program, in patients with type 2 diabetes.
https://www.sciencedirect.com/science/article/abs/pii/S0965229916300346 -
-
Fasting enhances extinction retention and prevents the return of fear in humans
Fear is prone to return following extinction that is the basis of exposure therapy for fear-related disorders. Manipulations that enhance the extinction process can be beneficial for treatment. Animal studies have shown that fasting or caloric restriction can enhance extinction and inhibit the return of fear. The present study examined the effects of fasting on fear acquisition, extinction, and the return of fear in humans. One hundred and twenty-five male participants were randomized into a fasting group and food group and exposed to a Pavlovian fear conditioning paradigm. Changes in plasma cortisol and ghrelin levels were examined using enzyme-linked immunosorbent assays. One-night fasting had no effect on fear acquisition but enhanced fear extinction retention and prevented the return of fear, and this effect persisted for at least 6 months. This procedure was also effective for remote fear memory. Plasma ghrelin levels were elevated after fasting and had a negative relationship with the fear response in spontaneous recovery test. However, overnight fasting did not affect cortisol levels. These findings indicate that fasting enhances extinction retention and prevents the return of fear, without influencing fear memory formation. We propose that this novel procedure may open new avenues for promoting extinction-based therapies for fear-related disorders.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6177454/The Association of Posttraumatic Stress Disorder with Fast Food and Soda Consumption and Unhealthy Weight Loss Behaviors Among Young Women
Results
PTSD symptoms were associated with an increased frequency of consumption of fast food and soda as well as unhealthy dieting behaviors but not with increased body mass index (BMI).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3153863/ -
@AlphaCog said in Metformin reduces gluconeogenesis that is typical of "fight or flight" stress.:
Association between Metformin Treatment and Improved Symptoms of Post-Traumatic Stress Disorder
One side of the problem.
Side effects of metformin
Haidut has posted:
The main first-line of therapy for diabetes II is the drug metformin, which happens to be a potent inhibitor of complex I of the electron transport chain (ETC) and as such is likely to drastically increase ROS levels as well as lipid (PUFA) peroxidation. Luckily, the pro-dopamine and anti-serotonin drug bromocriptine has also been approved by the FDA for diabetes II treatment and is a much better choice for both diabetes and AD.
Source:
PUFA/endotoxin cause Alzheimer Disease (AD), the plaques beta-amyloid/tau are protective
https://bioenergetic.forum/topic/2845/pufa-endotoxin-cause-alzheimer-disease-ad-the-plaques-beta-amyloid-tau-are-protective/1 -
@LucH Thanks for the link. I'm curious regarding the interpretation of Beta-Amyloid and Tau Proteins by various parties. The behaviour of the proteins seemed to have commonality with Uric Acid.
Metformin prevents p-tau and amyloid plaque deposition and memory impairment in diabetic mice
Abstract
Insulin deficiency or resistance can promote dementia and hallmarks of Alzheimer's disease (AD). The formation of neurofibrillary tangles of p-TAU protein, extracellular Aβ plaques, and neuronal loss is related to the switching off insulin signaling in cognition brain areas. Metformin is a biguanide antihyperglycemic drug used worldwide for the treatment of type 2 diabetes. Some studies have demonstrated that metformin exerts neuroprotective, anti-inflammatory, anti-oxidant, and nootropic effects. This study aimed to evaluate metformin's effects on long-term memory and p-Tau and amyloid β modulation, which are hallmarks of AD in diabetic mice. Swiss Webster mice were distributed in the following experimental groups: control; treated with streptozotocin (STZ) that is an agent toxic to the insulin-producing beta cells; STZ + metformin 200 mg/kg (M200). STZ mice showed significant augmentation of time spent to reach the target box in the Barnes maze, while M200 mice showed a significant time reduction. Moreover, the M200 group showed reduced GFAP immunoreactivity in hippocampal dentate gyrus and CA1 compared with the STZ group. STZ mice showed high p-Tau levels, reduced p-CREB, and accumulation of β-amyloid (Aβ) plaque in hippocampal areas and corpus callosum. In contrast, all these changes were reversed in the M200 group. Protein expressions of p-Tau, p-ERK, pGSK3, iNOS, nNOS, PARP, Cytochrome c, caspase 3, and GluN2A were increased in the parietal cortex of STZ mice and significantly counteracted in M200 mice. Moreover, M200 mice also showed significantly high levels of eNOS, AMPK, and p-AKT expression. In conclusion, metformin improved spatial memory in diabetic mice, which can be associated with reducing p-Tau and β-amyloid (Aβ) plaque load and inhibition of neuronal death.
https://pubmed.ncbi.nlm.nih.gov/34283253/Metformin attenuates Alzheimer's disease-like neuropathology in obese, leptin-resistant mice
Abstract
Diabetes increases the risk of Alzheimer's disease (AD). The pathological hallmarks for AD brains are extracellular amyloid plaques formed by β-amyloid peptide (Aβ) and intracellular neurofibrillary tangles consisting of hyperphosphorylated tau protein. This study was designed to determine AD-like brain changes in mice modeling for type 2 diabetes. The effects of metformin on these changes also were studied. Seven-week old male db/db mice received intraperitoneal injection of 200 mg kg− 1 d− 1 metformin for 18 weeks. They were subjected to Barnes maze at an age of 21 weeks and fear conditioning at an age of 24 weeks to assess their cognitive functions. Hippocampus was harvested after these tests for biochemical evaluation. The db/db mice had more tau phosphorylated at S396 and total tau in their hippocampi than their non-diabetic control db + mice. Activated/phosphorylated c-jun N-terminal kinase (JNK), a tau kinase, was increased in the db/db mouse hippocampus. Metformin attenuated the increase of total tau, phospho-tau and activated JNK. The db/db mice had increased Aβ levels. Metformin attenuated the reduction of synaptophysin, a synaptic protein, in the db/db mouse hippocampus. Metformin did not attenuate the impairments of spatial learning and memory as well as long-term hyperglycemia in the db/db mice. Our results suggest that the db/db mice have multiple AD-like brain changes including impaired cognitive functions, increased phospho-tau and Aβ as well as decreased synaptic proteins. Activation of JNK may contribute to the increased phospho-tau in the db/db mice. Metformin attenuates AD-like biochemical changes in the brain of these mice.
https://www.sciencedirect.com/science/article/abs/pii/S0091305712000640Gout risk in adults with pre-diabetes initiating metformin
Conclusions: Metformin use was associated with a reduced risk of gout among adults with pre-diabetes, suggesting that metformin may be important in lowering gout risk in individuals with pre-diabetes.
https://ard.bmj.com/content/early/2024/05/15/ard-2024-225652Metformin Lowers Serum Urate Levels but Not Gout Risk
https://www.rheumatologyadvisor.com/news/metformin-lowers-serum-urate-but-not-gout-risk/Effect of metformin use on clinical outcomes and serum urate in gout patients with diabetes mellitus: a retrospective cohort study
In conclusion we could not confirm a clinically relevant anti-inflammatory or urate lowering effect of metformin in patients starting ULT treatment and receiving usual care flare prophylaxis.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9153141/Metformin Initiation Cuts Gout Risk in Prediabetes
Metformin use lowers the risk for gout by 32% in individuals with prediabetes; however, the treatment doesn't change serum urate or C-reactive protein levels.
https://www.medscape.com/viewarticle/metformin-initiation-cuts-gout-risk-prediabetes-2024a1000a4jMetformin ameliorates high uric acid-induced insulin resistance in skeletal muscle cells
https://www.sciencedirect.com/science/article/abs/pii/S0303720716305482Metformin protects against insulin resistance induced by high uric acid in cardiomyocytes via AMPK signaling pathways in vitro and in vivo
https://www.biorxiv.org/content/10.1101/2021.01.29.428905v1 -
Could Alzheimer’s disease be a maladaptation of an evolutionary survival pathway mediated by intracerebral fructose and uric acid metabolism?
Although biological effects of fructose metabolism and its byproduct, intracellular uric acid, appear critical for the survival of many animals in nature, including our ancestors, in modern society, it appears to be overengaged, increasing the risk for metabolic syndrome, obesity, diabetes, and other conditions
...
uric acid translocatess NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) to the mitochondria, leading to oxidative stress that blocks the citric acid cycle (via inhibition of aconitase) and fatty acid β-oxidation. As mitochondrial function slows, glycolysis takes over, while uric acid inhibits AMP-activated protein kinase, reducing the ability to recover ATP. The effect is a reduction in ATP in the cell, activating a survival switch that includes hunger, thirst, foraging, fat accumulation, and insulin resistance.
...
Uric acid translocates NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) to the mitochondria, where it causes oxidative stress, reducing fatty acid oxidation (blocking enoyl CoA hydratase) while inhibiting aconitase in the citric acid cycle
...
In nature, dietary fructose from excessive intake of fruit provides a major pathway to activate this survival response, much like what occurs in the autumn when bears prepare for hibernation. However, fructose is also produced in the body via the polyol pathway, in which glucose is converted to fructose. The rate-limiting enzyme in the polyol pathway is aldose reductase, and its activity is stimulated during times of stress, such as when nutrient delivery is impaired (hypoxia or ischemia), when water supplies are low (dehydration, hyperglycemia, and hyperosmolarity), or when uric acid levels are high (reflecting degradation of nucleotides and ATP, suggestive of an energy crisis)
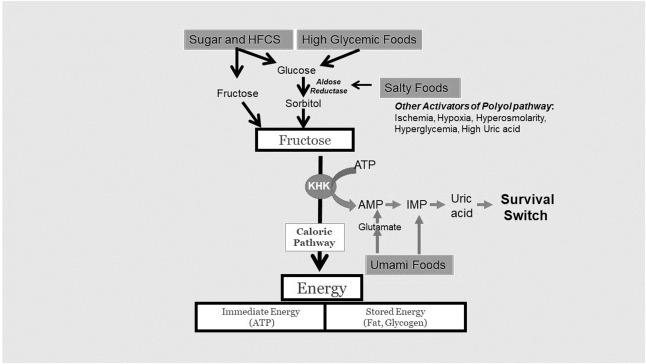
...
However, the studies that evaluated the differences between fructose and glucose in cerebral metabolism using BOLD MRI were performed early (∼15 min), thus making it more likely to reflect true differences between fructose and glucose. The striking finding from these studies was that fructose reduced blood flow to the posterior cingulate cortex, the hippocampus, the thalamus, and the occipital cortex; however, blood flow increased to the area of the visual cortex associated with food reward. Cortical blood flow also decreased. Fructose administration also stimulated hunger and desire for food. These responses are consistent with a stimulation of the foraging response. In contrast, glucose inhibited blood flow to the hypothalamus, thalamus, insula, anterior cingulate, and striatum while stimulating blood flow to the cortex. These responses are expected to inhibit not only the foraging response but also responses involving appetite and reward.
...
The brain can generate and metabolize fructose
Our hypothesis suggests that local fructose generation and metabolism may be the critical factor for how fructose induces AD because under normal circumstances, only 1%–2% of ingested fructose reaches the brain. Indeed, the brain is capable of producing fructose. As mentioned earlier, simply raising blood glucose levels increases brain fructose levels in healthy humans. Raising serum osmolality in mice by dehydration or salty food also stimulates fructose production in the brain (hypothalamus). Dietary fructose may also increase fructose production in the brain, possibly by raising uric acid levels in the brain. For example, acutely raising serum uric acid increases uric acid in both the hypothalamus and the hippocampus in association with local inflammation. In turn, uric acid stimulates fructose production and metabolism.
...
Fructose is elevated in the brain of patients with AD
Sorbitol and fructose levels (both components of the polyol pathway) were significantly elevated, averaging 3–5-fold higher in all regions of the brain studied, including the hippocampus, entorhinal cortex, middle temporal gyrus, cingulate cortex, sensory and motor cortex, and cerebellum.
...
Numerous studies have reported that subjects with AD have low serum uric acid levels, suggesting that this might be important to the pathogenesis. However, although serum uric acid may reflect fructose metabolism, it also is a general marker of nutrition status. Clinical manifestations of AD are often preceded by significant weight loss. Which may account for the lower serum uric acid levels on presentation of AD. This may also explain why obesity predicts AD in midlife but actually protects from AD late in life
...
https://www.sciencedirect.com/science/article/pii/S0002916523000047 -
@AlphaCog said in Metformin reduces gluconeogenesis that is typical of "fight or flight" stress.:
Fructose is elevated in the brain of patients with AD
Sorbitol and fructose levels (both components of the polyol pathway) were significantly elevated, averaging 3–5-fold higher in all regions of the brain studied, including the hippocampus, entorhinal cortex, middle temporal gyrus, cingulate cortex, sensory and motor cortex, and cerebellum.Agree on the observation but most diabetics take sweeteners. Polyol family. The liver has limited capacity to process polyols. Supposed to be considered residue, not to mention fermentation issues and the impact on the microbiome. No wonder in the long run there is an impact on the brain.
RP considered fructose as a balancing and metabolic element provided that there is a balance between fructose and glucose and that we remain under 50 grams of fructose. 25 gr in case of liver problem (and therefore diabetes).
Conclusion: this kind of study doesn't prove anything for me if the parameters are off the line. -
Elevated fructose in the brain is a sequester to protect it because of AD. Much like the protein plaques, which have been erroneously blamed as a result of AD.