What's up with the EGCG health potion trending on RPF?
-
@ThinPicking said in What's up with the EGCG health potion trending on RPF?:
Hey now, hey now
Thanks for remind:

Youtube Video -
@Korven some benefits at low dose but its toxic in the 100s of miligrams over time, supplement doses
(& ironically low copper makes mammals more vulnerable to it)
(similar thing to high dose quercetin probably, where its chelating too much copper over time for diet to match. plus the ROS damage from excess)https://pubmed.ncbi.nlm.nih.gov/25975988/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5943924/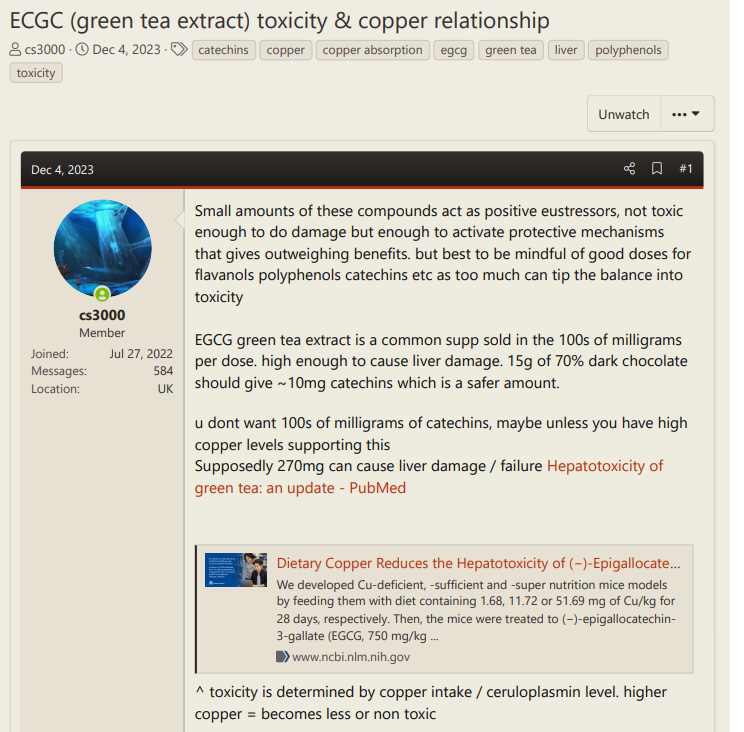



But not great to go high copper to prevent that as can cause other problems from the excessive copper , "low" mg doses of catechins should have some benefits
-
@cs3000 Thanks for the warning, indeed looks like there are quite a few case reports of liver toxicity from EGCG.
I already knew this formula was insane (upwards of 1 g EGCG per day, 3 mg molybdenum and more) but I was kinda curious what people thought of it
-
@cs3000
How would this study "Dietary Copper Reduces the Hepatotoxicity of (-)-Epigallocatechin-3-Gallate in Mice"
https://pubmed.ncbi.nlm.nih.gov/29295524/"Following EGCG treatment, the survival rates were 12.5%, 50% and 100% in the Cu-deficient, -sufficient and Cu-super nutrition groups of mice, respectively."
"suggest that Cu can relieve EGCG hepatotoxicity, possibly by up-regulating ceruloplasmin activity, which can be used to promote EGCG applications."
fit in with any of the EGCG narratives of the latter binding iron and hence freeing up copper from iron-copper binding as currently discussed on the RPF?
It kind of says the opposite, i.e. EGCG application requiring additional copper for survival.
On the other hand the used dosage (EGCG, 750 mg/kg BW) seems gigantic and maybe there's another compensatory effect at play there fore bare survival.
Or the iron/copper binding molecules in mice could have slightly different structures than their human equivalents? -
@CrumblingCookie not aware of the narratives but human toxicity dose was 270mg & 400mg so didnt need that higher dose
some polyphenols chelate copper more than iron , copper can be damaging in high amounts but also vital for mitochondria so if someone over focuses on eliminating it their mitochondria suffers
(TSH starts to rise in some studies that use 100s of milligrams of some polyphenols too , maybe binding too much iron copper zinc so they cant be used for regular functions enough. i guess copper is especially vulnerable because lower amounts / intake. e.g too much quercetin is detrimental to complex IV activity in mitochondria alongside ceruloplasmin :Quercetin is an iron & copper chelator
it lowers ceruloplasmin 50% , injected in mice at 50mg/kg mice dose
, In the present study a decreased activity of Complex IV in mice treated with quercetin is clear. This is well correlated with decreased levels of ceruloplasmin. {copper protein}
Our previous research [30] exposed that mild copper deprivation in mice is correlated with a decreased protein expression and activity of Complex IV at the level of OXPHOS supercomplexes along with a decrease in the ceruloplasmin levels -
@cs3000 said:
not aware of the narratives
In brief the current narratives of that RPF thread are that EGCG chelates iron from iron-copper complexes, which raises copper, which needs molybdenum to antagonize, chelate and excrete through increased uric acid through Mo-dependent xanthine oxidase, all of which needs vast amounts of vitamin B6 to replenish circulating copper with tissue copper, which needs vast amounts of biotin, which needs balancing with large amounts of vitamin B5 because of their shared transporter and lots of vitamin C, depending individually, to activate B vitamins and to keep iron and copper mobilized in circulation. All that so age-dependent iron and copper stores decrease and their alleged inhibitions are replaced with supplemented zinc ions to restore functions.
I've now had a brief look into ECGC + iron and it does appear to form (Ngal-)EGCG-iron complexes and alleviate iron-dependent injuries and ferroptosis. In this study, e.g. they beneficially used "only" 10mg/kg EGCG "EGCG activates Keap1/P62/Nrf2 pathway, inhibits iron deposition and apoptosis in rats with cerebral hemorrhage".
The cited mice study in which 750mg/kg EGCG was used is probably highly problematic. Probably the binding affinities and or the priorities regarding iron and copper shift and are different from much much lower high doses of EGCG as in 10mg/kg in rats. -
In this human study 300mg EGCG bound about 3-5mg (27%) of orally given iron but only 3% of intravenously given iron.
With only 150mg EGCG, curiously, there was 15% decreased retention of intravenously given iron. I.e. EGCG bound circulating iron but only when 150mg and not when 300mg EGCG were given at a time.
It's all a bit odd.
In this rat study the authors concluded "that green tea extract (GTE) ameliorates iron overload induced hepatotoxicity, apoptosis and oxidative stress in rat liver via inhibition of hepatic iron accumulation".I'm now somewhat curious about this topic because of liver issues and taking any zinc causing extra nausea and headaches and insomnia.
By this currently trending RPF thread this should imply I had both too much iron and copper. Or mainly too much iron in case molybdenum doesn't improve such effects by zinc.
Which when I think about it becomes more interesting in the context of high ferroptosis observed in the causative chain of neurodegenerative diseases. -
I have found this old gem in the 2016 - 2019 (2023) Molybdenum, Hard To Pronounce, Harder Still To Obtain thread on the RPF:
"A male health practitioner in his late thirties (the patient), apparently in full health, wanted to be even the healthier. His colleague suggested that he should try a molybdenum supplement (Molybdenum Chelated, 100 μg molybdenum per tbl., Nutriwest Co., Douglas, WY) to cure his »allergy« to perfume of his patients. That far he had had a remarkably empty medical record and was outstanding in his educational curriculum. According to his wife’s testimony, he was »easy going, doesn’t get angry often, and it takes a lot to rattle him«. The patient consumed a HCl supplement for »indigestion«, and other non-noxious nutriceuticals (Table 1) before he started to take molybdenum on 1 July 1997. The manufacturer’s recommendation read: »One tablet per day, or as directed«. Instead, the patient followed the recommendation from his physician »Take as needed« and consumed an average of 7 to 8 tablets of molybdenum per day (700–800 μg). He did not start with 700 μg; he started with about 300μg a day, and then gradually increased the intake over a period of 18 days. On day 7 he showed the first signs of anxiety and agitation. On day 14 he became mildly psychotic and experienced visual and auditory hallucinations. He also exhibited excessive craving for salt; so much so that he woke his spouse up at 2:00 a.m. to get him salt from a grocery store. On day 18 he could »smell the molybdenum all around him« and he abandoned molybdenum supplements. The smell was like the one of salt water, distinctive but mild. By the time the patient stopped taking molybdenum, he had already consumed a total cumulative dose of 13.5 mg of molybdenum. On day 19 he had severe psychosis with strong audio and visual hallucinations, insomnia, intense craving for salt, diarrhoea, and painful and cold extremities. On day 22 his hallucinations were accompanied by petit mal seizures and on day 24 he tried to take his life with a knife and, »chased by the devil«, he ran through a plate glass window and jumped headlong off a six-metre wall. The flight ended up with multiple body contusions and difficulties in movement. He struggled with an officer when the police picked him up, as he thought that they were trying to hurt him."
@Amazoniac 's comment on that back then:
Nice. This is what a true detox phase should look like.
Thanks for this, Amazoniac. It made me laugh.
I'm not too worried about it, he was taking a lot of other stuff that could've created a favorable environment for trace mineral toxicity. As always, most of these reports involve people taking more than necessary, insisting despite bad reactions for too long, and including a cocktail of other questionable compounds.
I agree with that. That guy had also taken various glandular extracts, betaine (which spares methionine demethylation to gain cysteine and further downstream sulfur metabolites incl. crucial sulfate), lots of bone meals (heavy metals? copper overload?). Whereas he had taken no B6, no extra sulfur, no extra zinc.
-
This looks good for higher molybdenum, higher zinc, higher cobalamin, sufficient selenium and lower copper levels:
Results: Plasma Se levels [and Mo levels] were significantly negatively associated with DunedinPACE*, whereas Cu levels were significantly positively associated with it.
*: DunedinPACE is a novel blood test that measures the pace of biological aging based on DNA methylation. It shows high reliability, predicts morbidity and mortality, and can be used to evaluate geroprotective interventions.
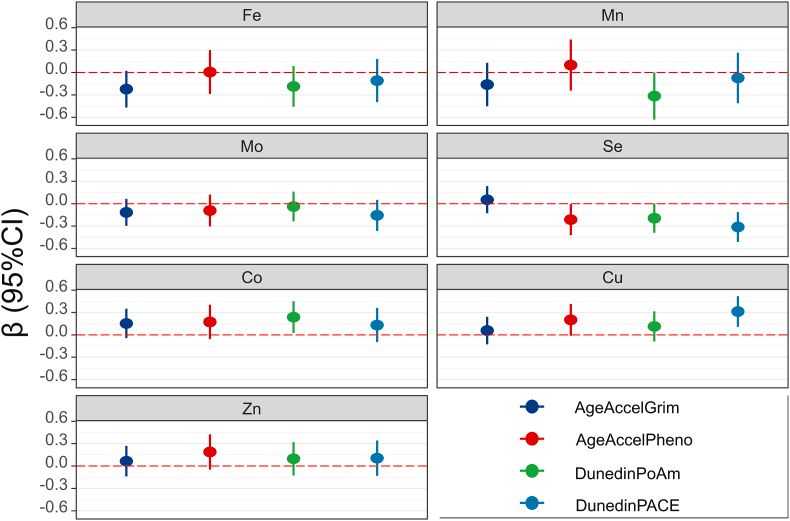
And this Chinese study finds the lowest metabolic syndrome at molybdenum plasma levels between 2-3 mcg/L, much above the typical lab reference range with an UL at only 1.3 or 1.4mcg/L. The latter is achievable with about 70mcg Mo daily. In the study with the 1490mcg Mo/day volunteer the plasma level was 5-6mcg/L.
Inverse Association of Plasma Molybdenum with Metabolic Syndrome in a Chinese Adult Population: A Case-Control Study
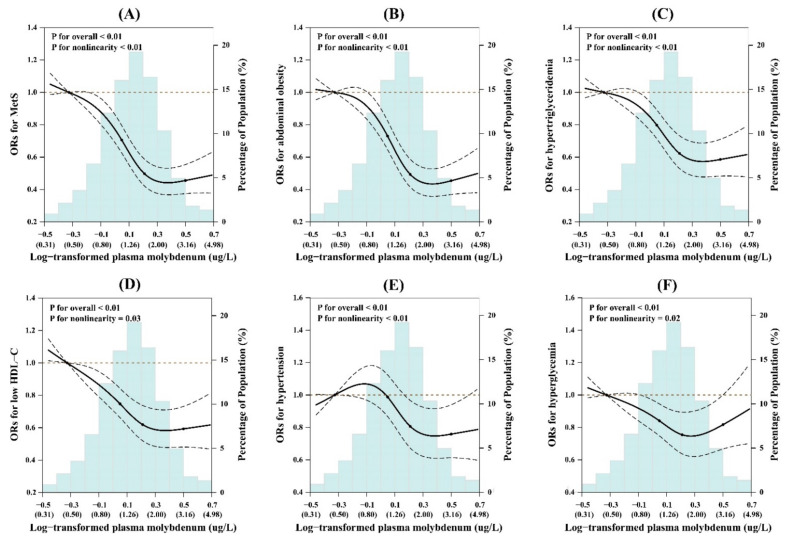
And this mice study finds serious thymus gland atrophy and disordered development of T cell subsets (viral infections, excess autoimmune response anyone?) in low molybdenum diets:
iTRAQ-based quantitative proteomic analysis of low molybdenum inducing thymus atrophy and participating in immune deficiency-related diseases -
sounds like that place is a shadow if its former self
-
@castle is there any other flourishing community/forum similar to it?
-
Excellent anon, anon.
What kind of a question is that!?
-
@Korven my first impression is any benefits he thinks he’s getting from the formula could be from the mega dosed biotin improving glucose metabolism and maybe the mega dosed vitamin c acting as an electron carrier. An energized liver should be producing all the ceruloplasmin and transferrin that is needed to regulate copper and iron to be utilized properly. Adequate atp being produced in cells should be optimizing zinc utilization at much lower levels than the 100mg+ his formula calls for.
So any strategy to increase oxphos should have a good effect on copper, iron and zinc homeostasis. Whether this formula has any special advantages over others or if it’s detrimental in many cases will probably be apparent within the next month or two as the forum members going all in should be honest enough about the effect it’s having on them and some are doing blood/hair tests to get some picture of what it’s doing.
The fact that this Heisenberg fellow still likes to do his formula daily or even multiple time daily to maintain what he thinks are the main benefits suggests it probably is the biotin and vitamin c effects on metabolism that he is feeling and not so much the chelation/zinc effects. He’s uninterested in blood tests of any kind so all we have is his word.
-
@gloryus I didn't have it on my radar that biotin is strongly needed for B6 activity. When I had read those high doses of biotin I though these guys are going to get crazy carb cravings.
However
indeed I've noticed that biotin+P5P leads to decreased hunger for me. Whereas biotin alone drives it up a lot. I appreciate to have been made aware of this connection.It's super wrong, though, to regard pyridoxin merely as an inactive form of B6 instead of a dirty antagonistic precursor.
And I reckon the huge amounts of biotin are not needed per se but may be serving as a crutch for sulfur to feed the molybdenum-dependent sulfite oxidase.
Then there's another question: If an increase of uric acid through molybdenum-dependent xanthine oxidase is indeed important for renal copper excretion are all the people doing this going go get intense feeding frenzies for meats or mushrooms so that they can replete and maintain their nucleotides?
However I haven't seen anything on uric acid binding to copper, but only on uric acid being protective against cuproptosis i.e. copper-induced cell apoptosis together with NO. So does the uric acid only protect against the damage from (temporarily) increased free copper? And should arginine be taken along with it? -
They are double and tripling the doses and doing it multiple times per day now. I don't see this ending well. Any questioning of the reasoning of what they're doing is shot down as you being the crazy one.
-
Entertaining thread

-
@Razvan you're in it. You got unbanned?
-
Some ponderings on that current H G 7 craze on the RPF. I am wondering whether indeed the central and crucial part about it all is the EGCG. And all other substances merely serve EGCG so that it's well tolerated.
And the EGCG's main function, aside from the removal of the intracellular metallothionine deposits (I'd still want to read up more on this hypothesis), would be that of an DNMT inhibitor: Releasing silenced genes, reenabling and strengthening cellular and tissue level activity and differentiation.So, the concurrent zinc will be taken up by EGCG and carried into the cells instead of the zinc freely floating around in circulation and giving you headaches etc. Zinc stores would be repleted and spared as in absolute terms EGCG would mainly chelate metals other than zinc, like iron.
Some thoughts: My lazy ChatAI inquiry responds with:
"Studies indicate that under certain conditions (e.g., neutral to slightly basic pH), one molecule of EGCG can bind two zinc atoms (Zn²⁺)"
By this, theoretically, each 350mg of EGCG could bind to 100mg of elemental zinc at the very most.
However, "the protocol" states 100mg zinc for about twice to triple the amount of EGCG (750-1200mg). So fully saturating the EGCG with zinc (if at all possible) is neither desired nor a great thing to do.
If I am not mistaken, the picolinic acid zinc salt already acts somewhat as an ionophere for shuttling zinc into cells. The experimental protocol so far concludes that people aren't responding as well to zinc picolinate as to other forms.
I would therefore speculate that it's not essentially about the zinc getting into the cells per se but instead about saturating some degree of EGCG with zinc.
Either to reduce its reactivity outside the cells or to promote it carrying predominantly zinc rather than other metals into the cells, and carrying other metals rather than zinc outside of the cells. Whilst somewhow shuffling around all the metals.Maybe someone with more dedicated brain activity to spare can look into that more profoundly. I.e. the specific affinities of EGCG to metals. And the formation and possible routes of degradation of metallothionine stores.
And maybe find out whether EGCG actually degrades MTs absolutely, or whether it changes the MTs' overall metal storage composition towards more zinc and less of other toxic metals.
Or whether EGCG by induction of Nrf2 simply upregulates MTs even more, alongside iron-binding ferritin, so just makes things run more smoothly by getting the free metals out of the way rather than truly excreting them. -
@CrumblingCookie said in What's up with the EGCG health potion trending on RPF?:
Maybe someone with more dedicated brain activity to spare can look into that more profoundly.
I'm far too busy.
-
B bot-mod referenced this topic on
-
Here's an interview between Elwin Robinson (lurked RPF for awhile) and the creator of "HG7" (silly name given by some forum members)
Tried it for a week and liked how it made me feel. It's true what you may have read in the big thread: large doses of these vitamins/minerals by themselves would destroy you, but work great in the ratios provided on the forum.
Honestly no different than the many experiments done in the past on RPF. I mean, the way I found RPF was Haidut's thread on megadosing Vit. E going somewhat viral on other health forums lol.