@CurmudgeonApple They use same metabolic pathways and when combined they synergistic effect. Metformin + Berberine combination is superior to using only one medication.
Posts made by Aegean
-
RE: Metformin Promotes Osteogenic Differentiation and Prevents Hyperglycaemia-Induced Osteoporosis by Suppressing PPARγ Expressionposted in Literature Review
-
Metformin Promotes Osteogenic Differentiation and Prevents Hyperglycaemia-Induced Osteoporosis by Suppressing PPARγ Expressionposted in Literature Review
Targeting nuclear receptors for diseases looks like needs to be understood better by all of us
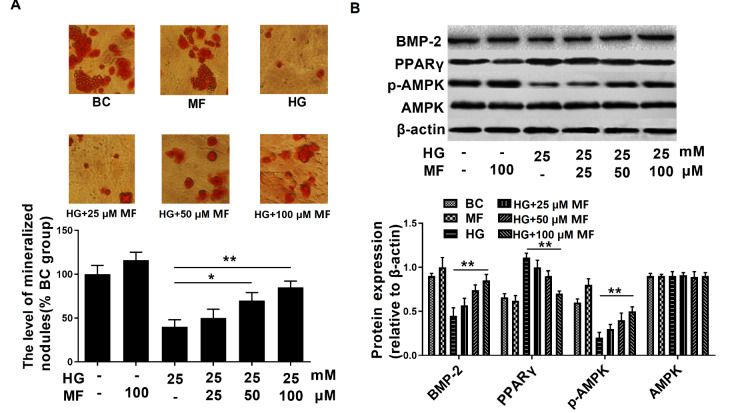
Metformin improves osteoblast differentiation and suppresses PPARγ expression in osteoblasts exposed to glucotoxicity :
To examine the effect of metformin on osteoblasts, we treated the streptozotocin-induced osteoporosis cell model with different concentrations of metformin (25, 50, and 100 μM). The results showed that 50 and 100 μM metformin significantly increased mineralization in hyperglycaemic osteoblasts. In the 100 μM metformin treatment group, the protein level of PPARγ was significantly decreased, and the levels of p-AMPK and BMP-2 were increased . These findings indicate that metformin improves osteoblast differentiation and suppresses PPARγ expression in osteoblasts.
PPARγ is highly expressed in the bone tissue of osteoporosis patients.
To confirm that PPARγ is the specific effector molecule of osteoporosis, we analysed the expression level of PPARγ in human bone tissue using published single-cell mRNA sequencing datasets. We found that PPARγ was highly expressed in preosteoblasts (1970/5729 cells) but was rarely expressed in mature osteoblasts (201/1528 cells) and was not expressed in other cells, such as chondrocytes, neutrophils and endothelial cells ). In addition, we analysed the PPARγ protein levels in the bone tissue of 30 T2DM patients, including 20 osteoporosis patients and 10 healthy individuals. The results showed that the PPARγ protein levels in osteoporosis patients were significantly higher than those in healthy controls. Thus, these results suggest that PPARγ is highly expressed in the bone tissue of osteoporosis patients and may play an important role in osteogenic differentiation.
PPARγ mediates the effects of metformin through the AMPK pathway
We examined whether PPARγ affects the activity of metformin through the AMPK pathway. Dorsomorphin (compound C) is an AMPK inhibitor. After inhibiting the activity of AMPK, we found that the protective effect of metformin was reduced: compared with those in the HG+MF group, osteoblast viability and mineralization, the secretion of ALP and OCN , and the expression of BMP2 in the HG+MF+Compound C group were decreased. Compound C also weakened the inhibitory effect of metformin on the expressions of pAMPK, PPARγ, PERK, ATF4, and CHOP in hyperglycaemic osteoblasts. These findings suggest that PPARγ affects the activity of metformin through the AMPK pathway.
PPARγ plays an important role in metformin-mediated effect on hyperglycaemic osteoblasts.
To verify that metformin improves mineralization by affecting PPARγ, we altered the expression or activity of PPARγ in a metformin treatment assay. In this assay, we altered PPARγ levels through overexpression and RNAi-mediated knockdown. The results showed that downregulating PPARγ levels improved the metformin-mediated effect on hyperglycaemic osteoblasts; osteoblast viability and mineralization, the secretion of ALP and OCN, and the expression of BMP2 were increased. Conversely, the increased PPARγ level in osteoblasts weakened the metformin-mediated effect on hyperglycaemic osteoblasts.
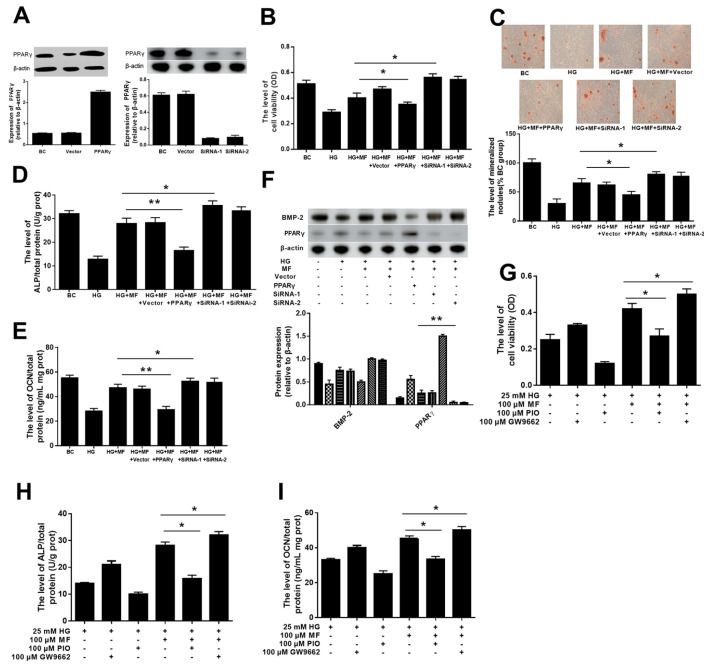
Results : In this study, we demonstrated that metformin enhanced osteoblast differentiation and downregulated the protein level of PPARγ in an osteoporosis cell model. Increasing the activity of PPARγ weakened these effects of metformin, whereas inhibiting the activity of PPARγ had the opposite effect. Additionally, we found that metformin regulated PPARγ function through the AMPK pathway. PPARγ mediates the effect of metformin via the ERS pathway. Furthermore, we found a relationship between the effect of metformin on bone metabolism and the activity of PPARγ in diabetic rats.
-
RE: Peaty Snacks & Dessertsposted in The Kitchen
@raypneat Spot fat reducing works with photobiomodulation. Conventional methods most likely will not affect regional fat tissue. Also topical absorbable solutions can modify lipid accumulation and oxidation in tissue.
-
PPAR Gamma Mediates High-Fat Diet-Induced Adipocyte Hypertrophy and Insulin Resistanceposted in Literature Review
PPARγ is playing major role in development in metabolic diseases. Biological differences in expression of PPARγ could be the important part when it comes to explaining susceptibility to metabolic diseases.
"... The expression of PPARγ has been shown to be sufficient to initiate adipogenesis in exponentially growing fibroblast cell lines, demonstrating a critical role for this receptor in the regulation of adipocyte differentiation. Embryonic fibroblast (EF) cells from mice lacking both C/EBPβ and C/EBPδ failed to express PPARγ and C/EBPα and were unable to differentiate in response to hormonal stimulation. EF cells from mice lacking C/EBPα failed to express PPARγ and were also unable to differentiate into adipocytes. Recently, ADD1/SREBP1 and PPARγ were reported to cooperate via the production of PPARγ ligand and stimulation of PPARγ expression by ADD1/SREBP1."
"Thiazolidinedione (TZD), a new class of antidiabetic drugs that increase insulin sensitivity, can directly bind and activate PPARγ and can stimulate adipocyte differentiation. We have previously shown that treatment of Zucker fa/fa rats with TZD caused an increase in the number of small adipocytes and a concomitant decrease in the number of large adipocytes that had produced an excess amount of TNFα and free fatty acids, thereby ameliorating insulin resistance. These results led us to propose that stimulation of PPARγ with potent synthetic agonists such as TZD may result in adipocyte differentiation and a consequent increase in the number of small adipocytes, thereby rendering the body more sensitive to insulin."
"PPARγ+/− showed normal weight gain and insulin sensitivity under a standard diet. To determine the role of PPARγ in HF diet–induced obesity and insulin resistance, we studied body weight gain and white adipose tissue mass of wild type and PPARγ+/− under either a high-carbohydrate (HC) diet or a HF diet. Body weight gain under a HC diet for 15 weeks was not distinguishable between wild type and PPARγ+/−. Under a HF diet, wild type gained significantly more body weight than that under a HC diet. In contrast, PPARγ+/− had little weight gain under a HF diet Gross appearance at sacrifice after 16 weeks of a HF diet revealed that fat mass in wild type under a HF diet was higher than that in both types of mice under a HC diet. PPARγ+/− were protected, at least in part, from the increased fat mass under a HF diet Moreover, marked fatty liver observed in wild type under a HF diet was again in part prevented in PPARγ+/−"
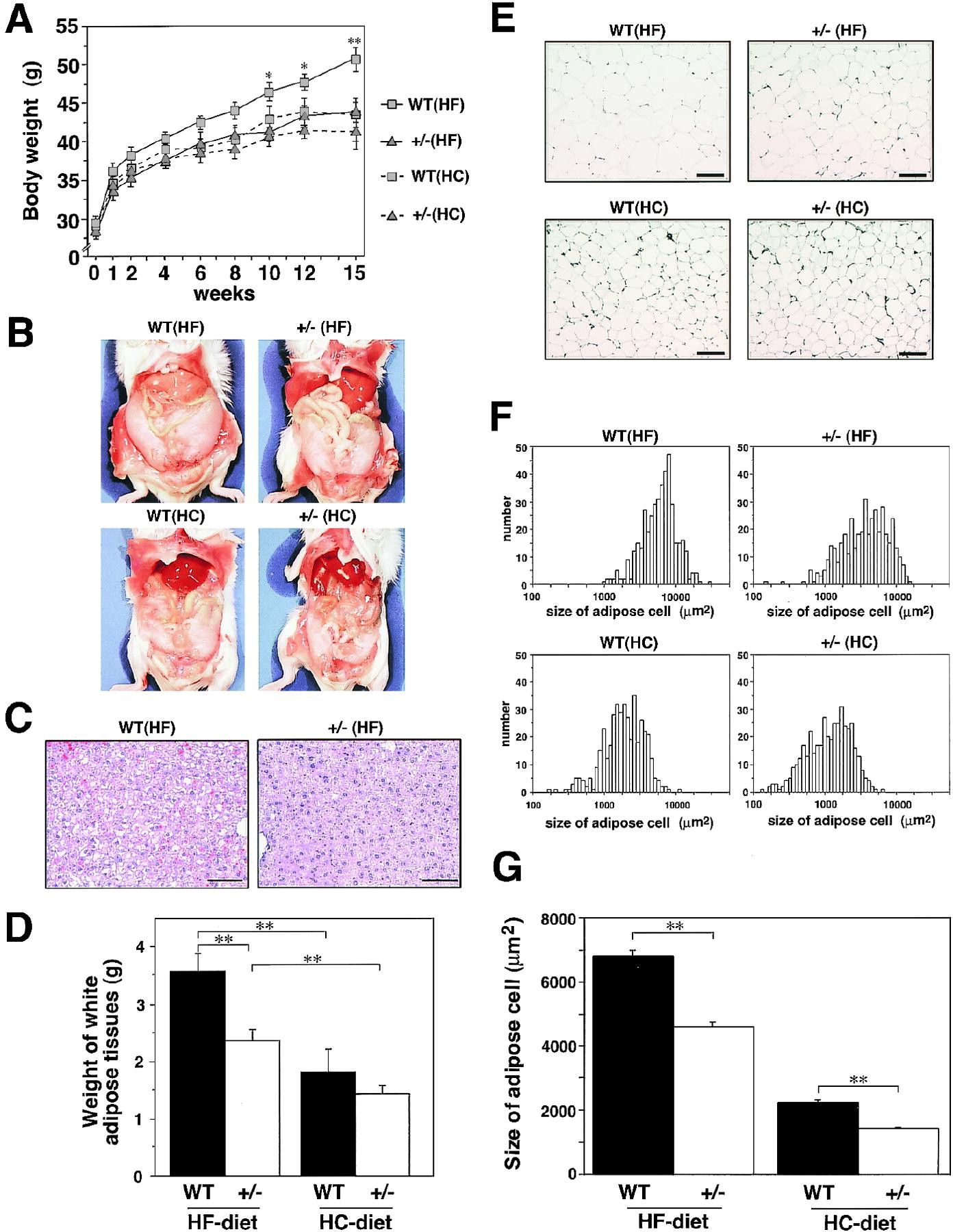 .
."PPARγ appears to facilitate energy storage under a HF diet in part by inhibiting expression of the leptin gene in adipocytes, the major hormonal regulator of energy homeostasis. It seems that PPARγ serves as a typical thrifty gene that provided a survival advantage in times of fast. However, in times of feast and sedentary life-style, such as today in Western countries and Japan, the two alleles of PPARγ appear to provide a survival disadvantage, causing an epidemic of hypertrophic obesity leading to serious obesity-linked morbidity and mortality. Finally, the identification of a novel role for PPARγ in obesity and insulin resistance should provide insight into the establishment of novel therapeutic strategies, such as PPARγ antagonist, for obesity and obesity-linked insulin resistance."