The Oxalate Content of a Food Can Mislead: Soluble and Insoluble Fractions
-
Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods
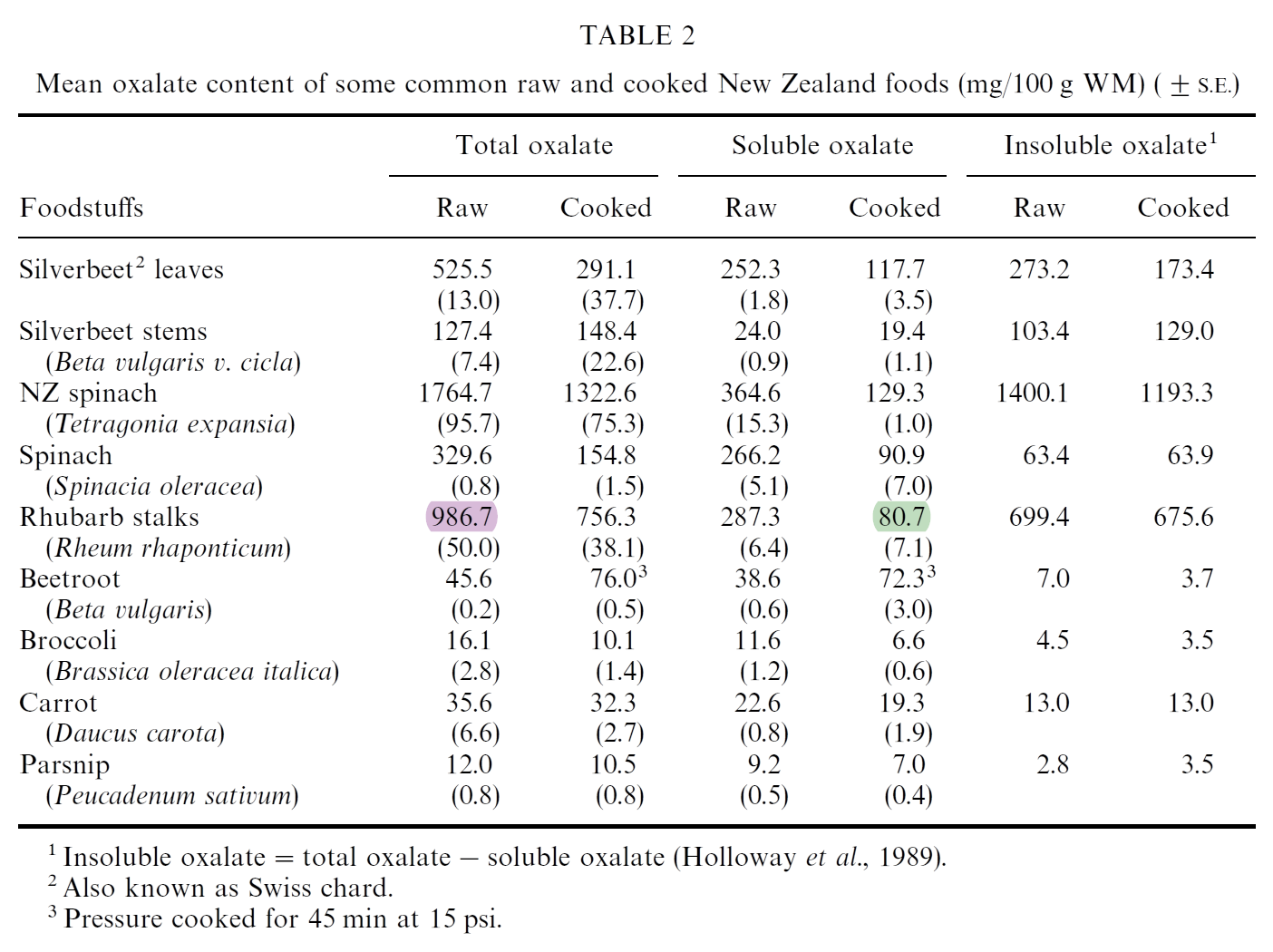
The difference after cooking is due to losses in preparation, and soluble oxalates are easier to leach. If the person doesn't mind wasting nutrients, boiling the food and discarding the water should reduce the exposure.
But what stands out is how insoluble oxalate predominates in foods, which is harder to absorb and prone to excretion. Taking extra calcium with the meal helps to form the insoluble calcium oxalate.
Calcium oxalate is (molar mass basis):
- 1/3 calcium
- 2/3 oxalate
As the stomach is emptied and the meal alkalinized, oxalic acid becomes charged and can complex with calcium. From the above, to complex with oxalate (and ignoring other interactions), calcium must be present in a solution in at least half of the soluble oxalate content.
- Soluble oxalate (mg) × 1/2 → Calcium (mg)
Even though the absorption of calcium is on average 2/3 of what's ingested, its absorption occurs after pancreatic alkalinization. At that stage of digestion, most of the calcium available in the meal can serve to complex with oxalate and not much is needed. Some calcium is also secreted into the duodenum along with sodium hydrocarbonate.
If a food has 100 mg of soluble oxalate, 50 mg of calcium may be enough to take care of it and can be considered to be wasted. The fraction of absorbable calcium will be the leftover of their reaction.
-
Milk with oat meal is always a good idea
-
I like to pair oxalate-rich foods with some source of calcium, but depending on the case (if you have recurring problems due to oxalate) it's better to pair with magnesium too, since magnesium oxalate is apparently 100x easier to excrete than calcium oxalate.
-
I love oxalates
-
This post is deleted! -
@Amazoniac I wonder if soaking in lye could help similar to how it breaks down starch in corn flour
-
The values above need to be revised. The doses of calcium needed in practice to complex with oxalate are higher than expected, and how calcium interacts with phosphate is relevant:
"The degree of interference reported here (~166 mg phosphorus for every 500 mg ingested calcium—0.4 mmol phosphorus for every mmol calcium) is substantially less than might have been predicted from simple stoichiometry. A 1:1 molar ratio for CaHPO4 would predict binding of 388 mg phosphorus by 500 mg calcium and, for Ca3(PO4)2 at a Ca:P molar ratio of 1.5:1.0, 258 mg phosphorus would be bound per 500 mg calcium. A molar ratio of 1:1 seems more likely at digestate pH values below 7.0. Even at the upper end of the confidence interval for the regression model—210 mg (6.8 mmol)—binding would still be substantially less than the lowest stoichiometric prediction. This discrepancy may be partly due to rapid absorption of phosphorus in the duodenum well before calcium and phosphorus complexes can form in the chyme. In any event it undoubtedly reflects the complexity and multiplicity of the interactions within the digestive residue."
(10.1080/07315724.2002.10719216)
Compare them:
- Predicted: Ca 1:1 P
- Practical: Ca 2.5:1 P
For oxalate:
- Predicted: Ca 1:1 Oxa
- Practical: Ca ?:1 Oxa
If we treat the interaction with oxalate as that with phosphorus (2.5:1) and adjust for mass (2.5:2), we would need 125 mg of calcium for every 100 mg of soluble oxalate.
One problem is guessing how much is soluble from the total oxalate content of a food. Based on the last two 'Cooked' columns (⇈), it's all over the place.
Another problem is the additional confounders. Member 'blabla' posted this elsewhere:
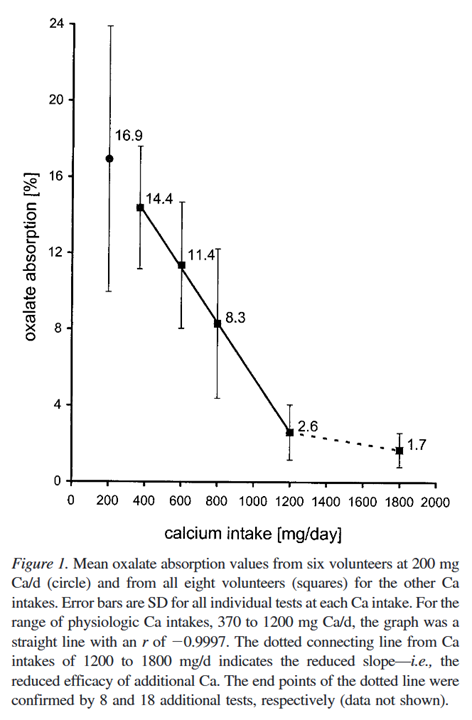
⠀(10.1097/01.asn.0000127864.26968.7f)- Up to 800 mg: dietary calcium alone
- More than 800 mg: dietary calcium and supplements (400 mg and 1000 mg)
Only the supplements were dosed with the labeled oxalate, suggesting that indirect interference is possible, but notice the degree of variations at low intakes. If you must supplement, it's preferable to dose in the same meal:
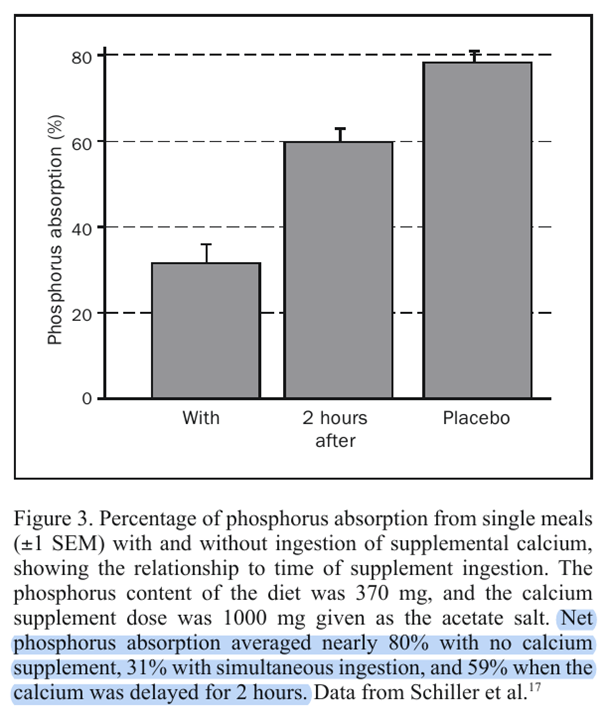
⠀(10.4065/79.1.91)But when the diet contains enough calcium (1000 mg), adding more seems unnecessary because a further reduction is minimal.