Conditional problems with vitamin A: a place for sane discussions
-
@Amazoniac in the plasma or in the liver????
-
They pride themselves on the fact that it's possible to put psoriasis into remission through poison A deprivation, but what about Coimbra's experiment who accomplished the same with "vitamin" D3 (875 mcg/d) and minor dietary modifications?
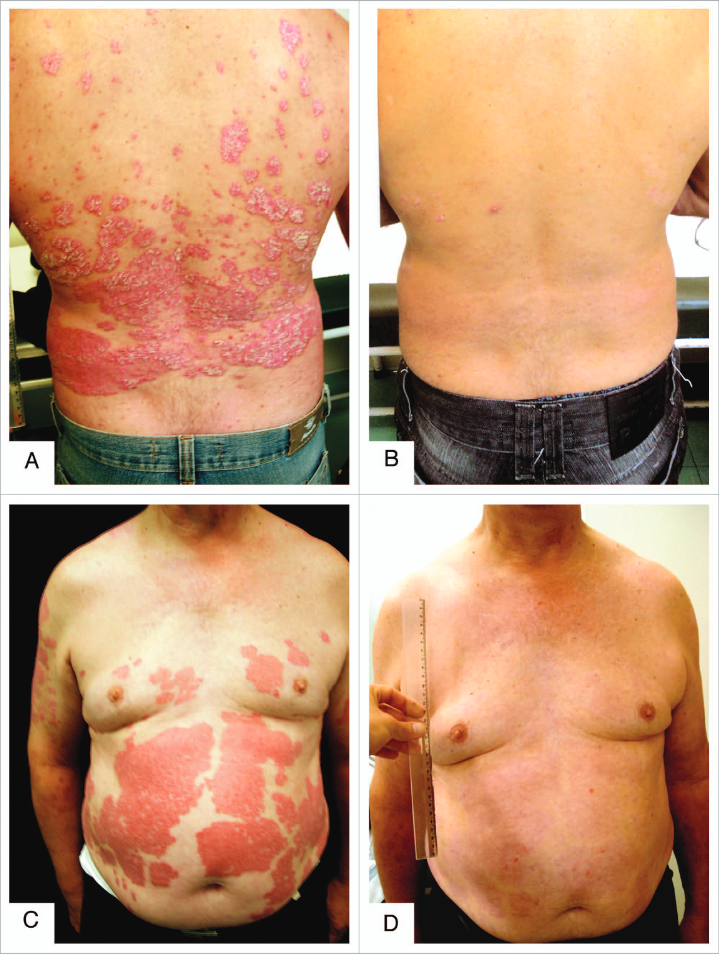
These approaches work, but are rudimentary. They manipulate upstream processes rather favoring targeting downstream specifics, that minimize the risk of having to compromise other functions to reach the therapeutic dose (for low or for high).
Psoriasis and beyond: targeting the IL-17 pathway | Nature
Even though targeting what's downstream is more sophisticated, the best thing to do is to try to address the pathogen above it all.
-
@thyroidchor27 said in Conditional problems with vitamin A: a place for sane discussions:
@Amazoniac in the plasma or in the liver????
That claim is based on what?
-
By the way, you can find various reports of 'undetectable' vitamin D levels; some because of analysis error (it can be challenging to quantify toxins that occur in modest amounts), but others not.
Controlling the availability of the precursor molecule can be a means to make up for an overactive pathway:
Undetectable serum calcidiol: not everything that glitters is gold
"Granulomatous hypercalcaemia is particularly sensitive to vitamin D administration even though toxic 25(OH) vitamin D levels are not reached [5]. This has been attributed to avid 25(OH) vitamin D metabolism into 1,25(OH)2 vitamin D by macrophage 1α-hydroxylase. Availability of 25(OH) vitamin D becomes the main regulator of 1,25(OH)2 vitamin D synthesis. Under these circumstances, treatment of vitamin D deficiency will increase the availability of 25(OH) vitamin D and lead to high 1,25(OH)2 vitamin D levels and hypercalcaemia [8, 9]."
-
@Amazoniac said in Conditional problems with vitamin A: a place for sane discussions:
They pride themselves on the fact that it's possible to put psoriasis into remission through poison A deprivation, but what about Coimbra's experiment who accomplished the same with "vitamin" D3 (875 mcg/d) and minor dietary modifications?
Have they presented any evidence regarding vitamin A deprivation and psiorasis, except tweets from good guy Garret Smith and the bible?
-
@Kvothe, I'm going by trust in the accounts of reliable people.
-
@Amazoniac Are there any in that crowd, and have they controlled their conclusions for other variables? The highly restrictive diets they eat exclude a huge lists of potential irritants that might cause auto-immune conditions and symptoms.
-
@Kvothe said in Conditional problems with vitamin A: a place for sane discussions:
@Amazoniac Are there any in that crowd, and have they controlled their conclusions for other variables? The highly restrictive diets they eat exclude a huge lists of potential irritants that might cause auto-immune conditions and symptoms.
'Maybe' to both questions. However, the accounts would be founded.
The Effect of Restricted Intake of Carotene and Vitamin A on Psoriasis Vulgaris
The exclusion of other toxins can contribute to the remission, but I don't think that the addition of isolated poisons to their current diets would lead to positive outcomes.
-
@Amazoniac Several people have shown me that paper when I asked for evidence that restriction of vitamin A improves psoriasis. I am sceptical. The authors note that most of the improvements and even complete clearing occured as early as 4/16 weeks after the start of the intervention. That's not nearly enough to significantly deplete retinol stores, especially on a diet that was low, but not insignificant in vitamin A, and it points to something more immediate.
Psoriasis is obviously not caused by excess vitamin A. People with psoriasis, in fact, tend to have lower levels of serum and hepatic retinol, and do not consume excess quantities of it. The underlying cause is found in other processes, and disturbances in the local retinol metabolites are an effect but not the cause of the problem. -
@Kvothe, a diet low in poisons leads to remission and returning with the normal diet results in relapse. Their supplementation without a change in diet does the same. Unless we assume that carotene metabolites are interfering with the action of poisonoic acids or it's disturbing the cell after being incorporated in fatty regions, we have to entertain the other options.
It wouldn't take a minimum of a month to notice the first positive effects from the exclusion of other irritants from the diet, but would match the decrease in circulating carotenes.
-
Cyclosporine is also an effective treatment for psoriasis. Given the extensive evidence of vitamin A’s role in immune function, I think it is more likely that psoriasis improvement with a depleting diet is a side effect of immune suppression instead of a root cause being discovered.
https://pubmed.ncbi.nlm.nih.gov/11375434
“Vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection, and by diminishing the function of neutrophils, macrophages, and natural killer cells. Vitamin A is also required for adaptive immunity and plays a role in the development of T both-helper (Th) cells and B-cells. In particular, vitamin A deficiency diminishes antibody-mediated responses directed by Th2 cells, although some aspects of Th1-mediated immunity are also diminished.”
-
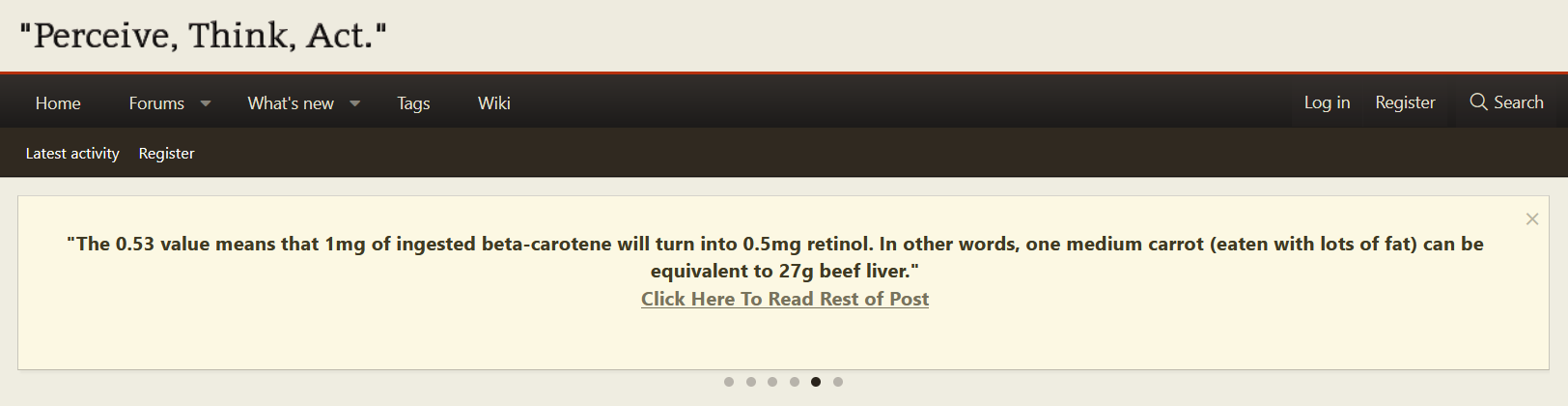
It directs to..
a) Professor Smith:
"If a person takes in more toxic "vit" A than they excrete on a regular basis, then they are ACCUMULATING it in their liver and bodyfat
If 306 mcg beta-carotene (that's 1020 IU, one carrot has 6000 IU!) took 58 DAYS to turn over...do you realize just how fast you are filling up with this toxin?
1 beta-carotene eventually has to split into 2 retinaldehydes"
b) Disciple:
"Wow.
And this is also a great study for showing how much beta-carotene is actually converted to retinol. They write a minimum of 62% is turned into retinol. So a lot will eventually go the retinol route.
"What is clear is that, by our estimates, a minimum of 62% of the absorbed β-carotene was cleaved to vitamin A and by this reasoning the vitamin A value of β-carotene dose was 0.53. [The calculation is as follows: Assuming central cleavage, 1 mol of β-carotene yields 2 mol of vitamin A. If 62% of the absorbed β-carotene were cleaved to vitamin A, 1 mol of β-carotene would yield 1.24 mol of vitamin A. Thus, the vitamin A value of the absorbed dose is 1.24. Yet, the dose was 42.6% absorbed. So, the actual vitamin A value is (1.24) (0.426) or 0.53.]"
1 molecule of beta-carotene turns into 2 molecules of "active vitamin A".
The 0.53 value means that 1mg of ingested beta-carotene will turn into 0.5mg retinol. In other words, one medium carrot (eaten with lots of fat) can be equivalent to 27g beef liver.
Due to the time-delayed conversion, an acute poisoning is not possible, but a chronic poisoning is."
Their claims:
- a) "[..]one carrot has 6000 IU!" (1,800 mcg)
- b) "[..]one medium carrot (eaten with lots of fat) can be equivalent to 27g beef liver."
Beef liver:
- Poisonol 10,000 mcg/100 g
Carrot:
- Alpha-carotene: 2,000 mcg/carrot
- Beta-carotene: 5,000 mcg/carrot
We can disconsider half of the alpha-carotene amount and treat the other half as beta-carotene:
Beef liver:
- Poisonol 10,000 mcg/100 g
Carrot:
- Beta-carotene: 6,000 mcg/carrot
The author of the second post seems to be into the topic of poison A for a while, yet for some reason acts as if the information presented in the mentioned experiment was novel. These equivalences are found in almost all textbooks, even the main sections of the dedicated Wikipedia page have them, suggesting that pure b-macabrotene has half of the activity of poisonol:
- 1 µg RAE = 1 µg retinol from food or supplements
- 1 µg RAE = 2 µg all-trans-β-carotene from supplements
Now the equivalences from their claims:
- a) 1 µg RAE = 3.3 µg of all-trans-β-carotene from carrots
- b) 1 µg RAE = 2 µg of all-trans-β-carotene from carrots
The disciple is basing his equivalence on the following values from the linked experiment:
- Absorption: 42.6%
- Conversion: 62%
- Total (42.6% × 62%): 26%
This is close to a quarter, that results in a mass equivalence of about poison 1:4 b-mac, not 1:2 as he's arguing.
The source of confusion is because he's applying a molar to a mass ratio. B-carotene can yield two poisonols, but the mass is going to remain similar after cleavage and metabolism, with minor gains from the incorporation of new elements (that I'm going to disregard).
To arrive on his value:
- 26% × 2 = 53% (0.53)
It would've been preferable for the disciple to state:
"The 0.53 value means that 1 mg of ingested beta-carotene will turn into
0.5mg~0.25 mg retinol." The factor accounted for the potential of b-carotene to yield two poisonols, so it has to be applied after we halve the amount.At this stage, we can already discard the idea that a carrot will poison you as an ounce beef liver.
The spinach used in the quoted experiment served to tag macabrotene, the plant assimilated the labeled carbon of CO2 from the environment, allowing them to track the poison. But when it was time to contaminate the victim, the macabrotene was isolated and given in purified form at a low dose.
The food matrix lowers the availability of toxins, making them more difficult to be extracted, which is why researchers define a marked drop in efficiency when the b-macabrotene is derived from foods:
- 1 µg RAE = 1 µg retinol from food or supplements
- 1 µg RAE = 2 µg all-trans-β-carotene from supplements
- 1 µg RAE = 12 µg of all-trans-β-carotene from food
If we ignore this factor and pretend that fibrous carrots are an oil, the amount used in the experiment was modest (300 mcg) and we know that the rate of absorption and conversion tends to decrease as the dose gets high.
But let's reject this too and assume that it remains uninhibited up to 6 mg or so. However, the adjustment is beyond the meal content, the degree of body contamination with the poison is another factor that determines its fate. It's why the purity of the victim matters to interpret the dosing response to carotenoids: contaminated and decontaminated persons will metabolize it differently. When the body is overloaded, the rate of conversion to retinoids is reduced. To a lesser extent, the same goes for their absorption. Yet, carotenoids can be excreted intact.
"1 beta-carotene eventually has to split into 2 retinaldehydes"
It doesn't. His own equivalence conflicts with this claim, even if we discount the unabsorbed fraction (and he probably didn't):
"1 beta-carotene eventually has to split into 2 retinaldehydes":
- 6,000 mcg b-carotene → about 6,000 mcg PAE
"1 [absorbed] beta-carotene eventually has to split into 2 retinaldehydes":
- 6,000 mcg b-carotene → about 2,500 mcg PAE
"[..]one carrot has 6000 IU!":
- 6,000 mcg b-carotene → about 1,800 mcg PAE
Which is it?
"If you take something more than you can excrete, you accumulate"
Of course.
"If 306 mcg beta-carotene (that's 1020 IU, one carrot has 6000 IU!) took 58 DAYS to turn over...do you realize just how fast you are filling up with this toxin?"
A long half-life doesn't result in an indefinite accumulation, only until a steady state is reached, when poisoning for profits should plateau with regular consumption. Something like this in dosing more often:
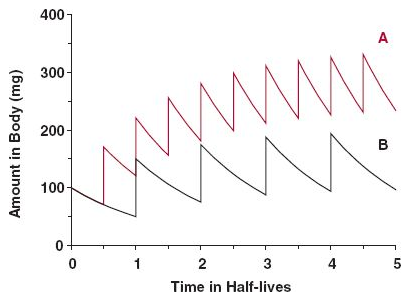
Source: the internet.Some accumulation is normal and healthy; or should we treat macabrotenoids as toxins to be nuked from the body at all costs? "Vitamin" E is a toxin too (Smith, 2023), but is it harmful when it's not detoxified fast enough to avoid a detectable level, leading to 'accumulation' to be associated with problems?
Consider this: you consume a meal, whose elimination would take 24 hours. Instead of waiting for this period to eat the next, you consume multiple meals in between (may be 4 in a day), that result in accumulation of matter, but without issues. The accumulation is temporary, purposeful and the body is adapted to it. It's not immediate, but the matter eventually leaves the body to the extent that it's consumed: no stuffing until explosion occurs because the input matches the output despite the delay.
Excess carotenoids or calories should come with signs. The early stages are benign, will indicate that the person has to cut back or improve the capacity to handle them. Yet, carotenemia isn't reliable to diagnose poison A toxicity because it can be caused for different reasons (such as lack of nutrients or poor protein synthesis). As an example, if a person needs to take supplemental thyroid hormones and suddenly stops the treatment, it can be followed by carotenemia.
The rate of storage and mobilization is adaptable as well. In case someone discontinues the consumption of poisons altogether, it can signal conservation and a longer persistence in the body. Therefore, the length is variable.
All in all, while we have people debating whether an inability to convert macabrotenes is common or a real concern for persons who don't consume preformed poisons, we have the poison A crowd alarming you that regular carrot consumption is a ticket to the hospital over time, for having the same potential of destruction as liver in the diet, neglecting the multiple steps that regulate these toxins as drugs. #toxicbileapocalypse
-
Gut commensals expand vitamin A metabolic capacity of the mammalian host
"Here, using liquid chromatography-tandem mass spectrometry (LC-MS/MS), we found that the presence of commensal bacteria resulted in a high concentration of the active retinoids, atRA and 13cisRA, as well as the principal circulating retinoid, ROL in the mouse gut lumen. Our work demonstrating gut bacteria’s VA metabolic capacity in generating multiple pharmacologically active retinoids lays the groundwork for developing bacterial retinoid therapy to treat diseases and tackle VA deficiency disorders."
"Cecum was chosen since it harbors the largest consortia of metabolically active bacteria in the digestive tract. To obtain a precise snapshot of flux in VA metabolites due to gut microbiome intrinsic VA metabolic activity, we compared the retinoid metabolomes from cecal contents from germ-free (GF), conventional (CV), and antibiotic treated mice (CV+Abx)."
"We saw that the cecal contents of GF mice had significantly lower concentrations of atROL, atRA, and 13cisRA compared to CV mice cecal contents (Figures 1B–D). This pattern was also observed along the length of the intestine (
Figures S1A–G). The observed differences in VA metabolites were not due to differences in dietary RE since both the GF and CV mice were maintained on similar autoclavable diet and had equal levels of dietary retinyl palmitate in their cecal contents (Figure 1E). Importantly, CV mice treated with cocktail of antibiotics in drinking water for 3 days showed drastic depletion in concentrations of all VA metabolites except for RE in the cecal contents compared to the control mice (Figures 1B–E), establishing that the presence of gut microbiome is required for high amounts of ROL, atRA and 13cisRA in the gut lumen. Taken together, our data suggest that members of the gut microbiome have the metabolic potential to process dietary VA into ROL and generate its active metabolites atRA and 13cisRA."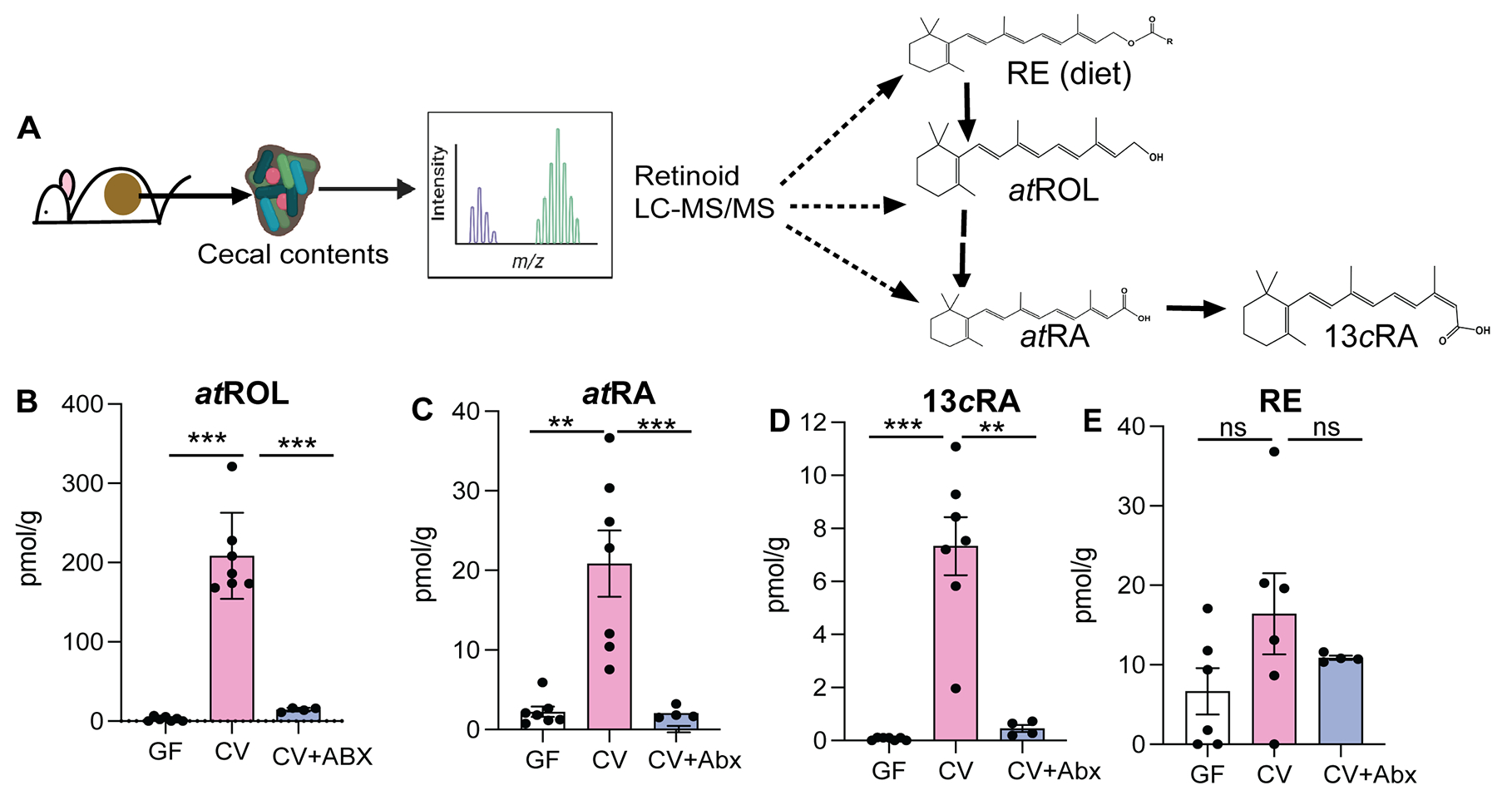
"Next, we investigated if VA metabolic activity observed in the gut lumen was correlated with presence of specific bacterial members. To determine the VA metabolic potential of different bacterial groups, CV mice were treated with either
- Vancomycin, which targets anaerobic commensals belonging to class Clostridia and Bacteroidia (Duncan et al., 2021), or
- Neomycin, which specifically targets aerobic commensals such as Proteobacteria (Hazenberg et al., 1983).
We observed that vancomycin treated mice had significantly lower ROL, atRA and 13cisRA concentrations in the gut lumen compared to untreated mice (
Figures 2A–C). Neomycin treatment did not affect retinoid levels in the gut lumen. The reduction in retinoids in the gut lumen of vancomycin treated mice was not due to reduced bacterial load (Figure S2C) suggesting that vancomycin sensitive commensal anaerobes provide the VA metabolic activity in the gut lumen.""To confirm the differential ability of commensal bacteria to metabolize VA, we colonized GF mice with Proteobacteria that bloom during vancomycin treatment (
Figure 2D). A second group of GF mice were given Clostridia spores in addition to Proteobacteria isolated from vancomycin treated mice. GF mice colonized with Proteobacteria alone did not show any increase and had comparable amounts of retinoids in the gut lumen as GF controls (Figures 2E–G). In contrast GF mice colonized with Proteobacteria and Clostridia spores showed significant increase in concentrations of atROL, atRA and 13cisRA in the gut lumen. 16S rRNA analysis of cecal community confirmed that gut microbiomes of Proteobacteria colonized mice lacked commensal anaerobes while mice that received spores in addition to Proteobacteria had high abundance of bacteria belonging to class Clostridia that restored all three retinoids in the gut lumen (Figure 2H).""We previously showed that gut bacteria can modulate VA metabolic machinery in the IECs and regulate RA amounts in intestinal tissue (Grizotte-Lake et al., 2018). To establish that bacteria intrinsic VA metabolic activity in the gut lumen is independent of host VA metabolic machinery, cecal contents from CV mice were harvested and grown in nutrients rich media and then supplemented with 10mM RE or ROL under anaerobic conditions. After 3 hrs., conversion of VA precursors into ROL, atRA and 13cisRA was quantified by LC-MS/MS (
Figure 3A). In the presence of bacteria, RE was hydrolyzed into ROL and ROL was oxidized to RA isomers (Figures 3B–F and S3). Bacterial cultures by themselves did not contain any detectable ROL or RE. The media alone supplemented with RE, or ROL did not yield quantifiable metabolites (Figures 3B–F) suggesting that there was no spontaneous conversion of RE and ROL into RA. These results establish that cecal bacteria can metabolize dietary VA precursors to active metabolites independent of the mammalian hosts and that gut bacteria encode the genetic machinery needed for performing VA metabolism." "Taken together, our results demonstrate that culturable consortia of gut bacteria can perform all the enzymatic reactions required for VA metabolism generating retinoid metabolites independently of the host.""Many Lactobacillus spp are naturally resistant to vancomycin (Swenson et al., 1990), and accordingly we observe that vancomycin treatment can result in bloom of Lactobacillus spp (
Figure S2B). We found that L. intestinalis, which has high ALDH activity was highly sensitive to vancomycin (Figure S4B) suggesting that loss of L. intestinalis upon vancomycin treatment could result in reduced retinoid metabolites in the gut. To evaluate if probiotic application of L. intestinalis to mice during vancomycin treatment would restore RA levels in the gut, mice were treated for 10 days with vancomycin and were given three applications of 108 CFUs of L. intestinalis or L. murinus. Similar bacterial loads were observed in all groups (Figure S4C). Retinoid analysis showed that L. intestinalis restored atRA levels of vancomycin treated mice while L. murinus, that does not show ALDH activity could not restore gut RA levels in vancomycin treated mice (Figure 4F)."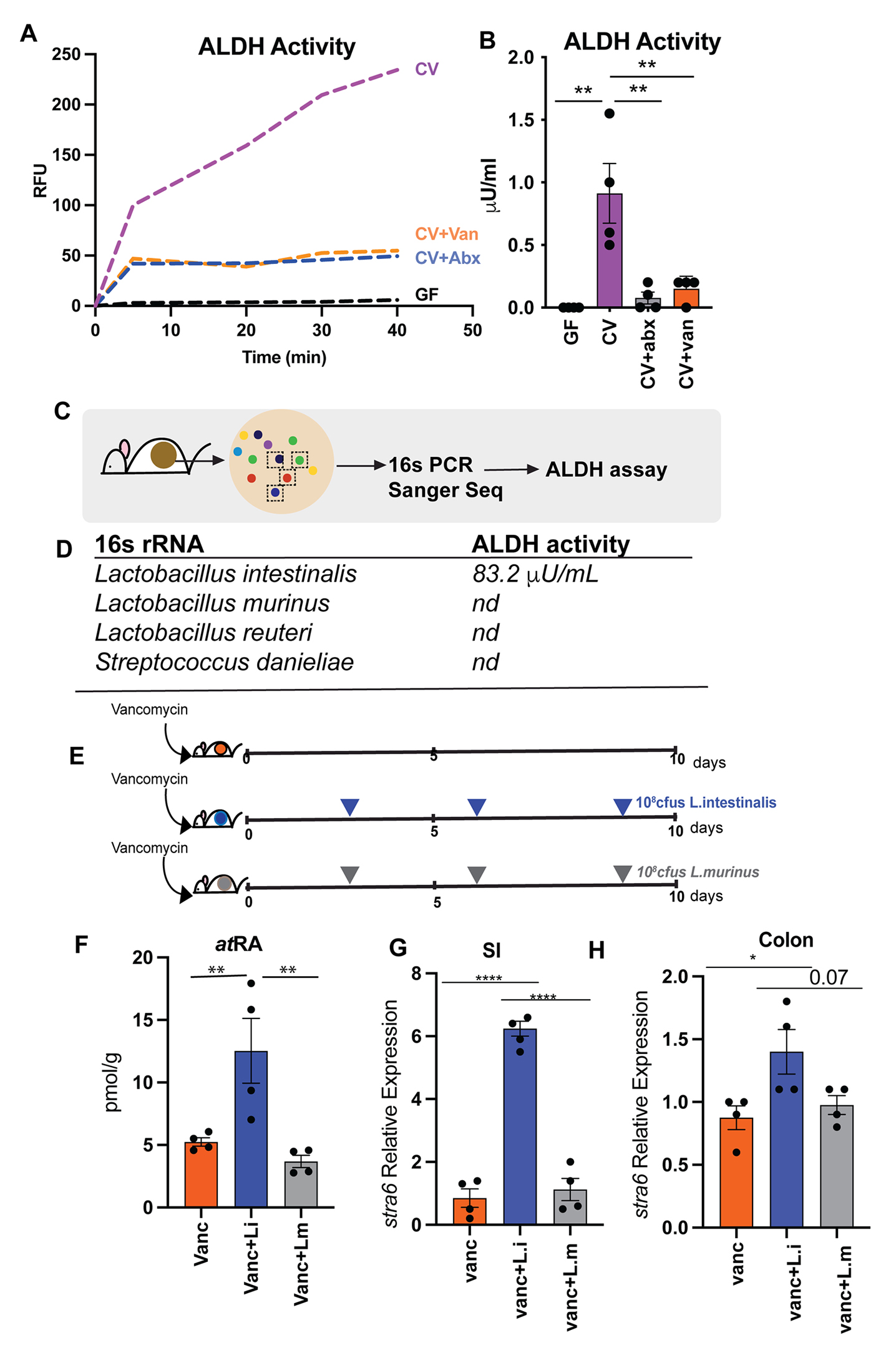
"[..]monocolonization of GF mice with either L. intestinalis, SFB or B. bifidum did not restore RA levels in the gut of GF mice (
Figure S4A) indicating that VA metabolism is an emergent property of gut microbiome and requires other members of consortia to participate in multistep conversion of VA to RA. In vancomycin treated animals L.intestinalis is able to restore RA production due to presence of vancomycin resistant bacteria that provide the metabolic machinery needed for other steps of VA metabolic pathway.""Next, we wanted to determine if restoration of RA in gut lumen following L. intestinalis treatment would induce RA responsive genes in the host. To do this we quantified known RA inducible host gene, Stra6 (Carrera et al., 2013; Kawaguchi et al., 2007). STRA6, has been shown to be essential in facilitating the cellular entry and exit of retinol and therefore is key in regulating how much retinol host cell can uptake. RA inducible gene stra6 was significantly induced in small intestine and colon of L. intestinalis supplemented mice compared to non-supplemented and mice gavaged with L. murinis (Figures 4G–H). We also observed higher expression of Nos2 gene [iNOS] in small intestine of the L. intestinalis supplemented mice in comparison to non-treated and L. murinis supplemented mice (
Figure S4D). The nitric oxide synthase 2 (nos2) gene was recently shown to be inducible by bacteria derived RA in the intestinal epithelium and its upregulation protected against pathogen colonization (Woo et al., 2021). We did not detect any significant differences in the pattern of inflammatory cytokines expressions in the small intestine and colon between the groups (Figures S4E–F) suggesting that addition of L. intestinalis is not linked to inflammation. Overall, our results demonstrate that bacteria with ALDH activity can restore RA following its depletion upon antibiotic treatment and promote the induction of RA responsive genes in the host." -
"Considering that the biological activities of isomers are lower than all-trans-retinol, the knowledge of retinol isomer profile in liver is useful for a precise estimation of safe dietary intake levels of animal liver."
"[..]when the determination can not separate retinol isomers, total retinol activity in animal liver products may be overestimated."
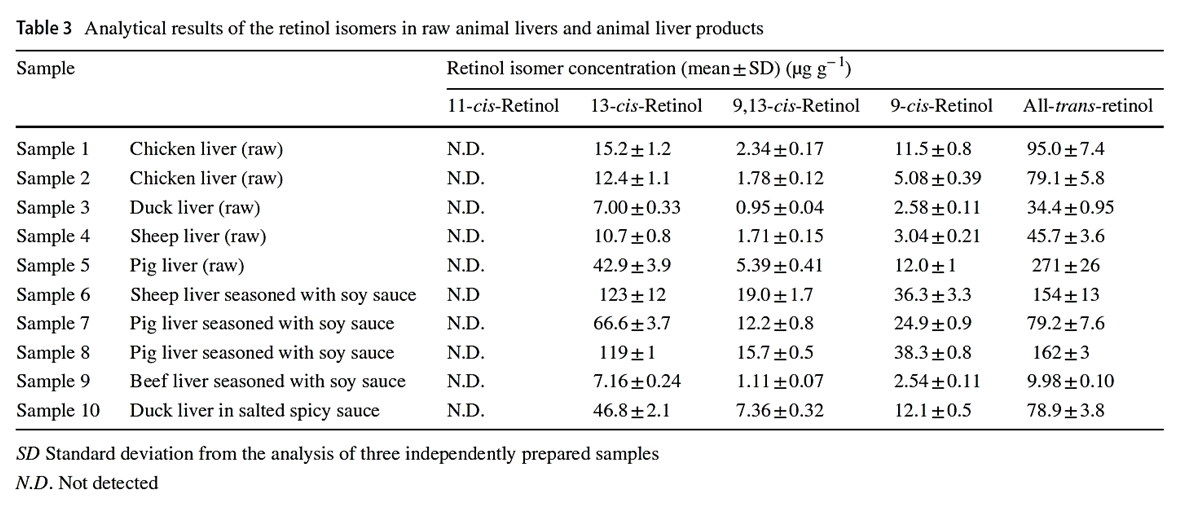
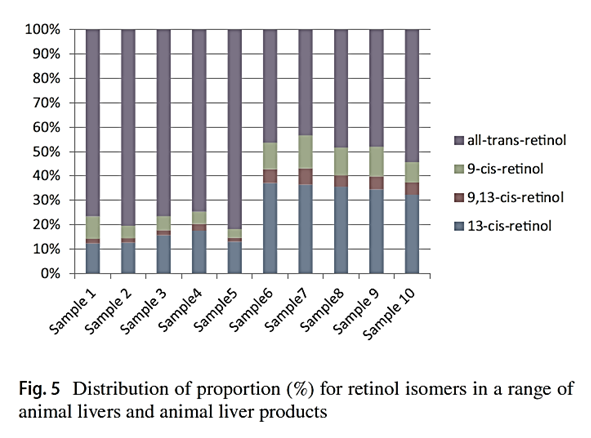
-
Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens
"Here we find in mice that in conjunction with IL-15, a cytokine greatly upregulated in the gut of coeliac disease patients[3,7], poisonoic acid rapidly activates dendritic cells to induce JNK (also known as MAPK8) phosphorylation and release the proinflammatory cytokines IL-12p70 and IL-23."
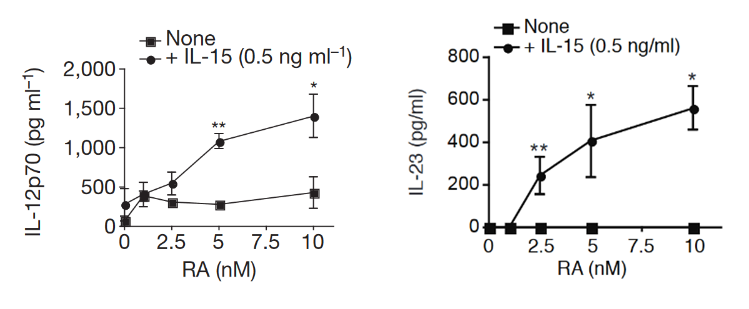
"Our study reveals that in the presence of IL-15, RA has unforeseen co-adjuvant properties that induce TH1 immunity to fed antigens (
Fig. 4f). It indicates further that under infectious conditions associated with induction of IL-15 and IL-6 in the intestinal mucosa, RA will also promote TH17 immunity. These observations caution against the use of poison A and RA for the treatment of autoimmunity and inflammatory intestinal disorders associated with high levels of IL-15. Indeed, a causal relationship between retinoids used for the treatment of acne and inflammatory bowel disease has been implicated in a subset of patients[25].""More generally, our study supports the concept that there are no ‘unconditional’ suppressive factors, and that integration of tissue and exogenous signals determine the class of the immune response, which ultimately needs to be tailored to the tissue and the antigen. In line with the idea that the same proinflammatory factors trigger different immunological outcomes depending on the tissue in which they are induced, we found that the ability of IL-12 to inhibit Treg-cell induction was blocked by butyrate, a metabolite produced by commensal bacteria present in the colon but not in the small bowel (data not shown)."
"Our observations may also explain why oral tolerance is disrupted in patients with inflammatory bowel disease[28] who also have dysregulated IL-15 expression in the gut[29]. Lastly, our results indicate that inhibiting IL-15 signalling may constitute a therapeutic intervention to restore mucosal tolerance to luminal antigens."
Insights into the Pathogenesis and Treatment of Psoriasis
"[..]dendritic cells and effector T-cells are important in the development of the psoriastic lesion, and cytokines produced by these cells stimulate keratinocytes to proliferate and increase the migration of inflammatory cells into the skin, promoting epidermal hyperplasia and inflammation (Monteleone, G. et al., 2011)."
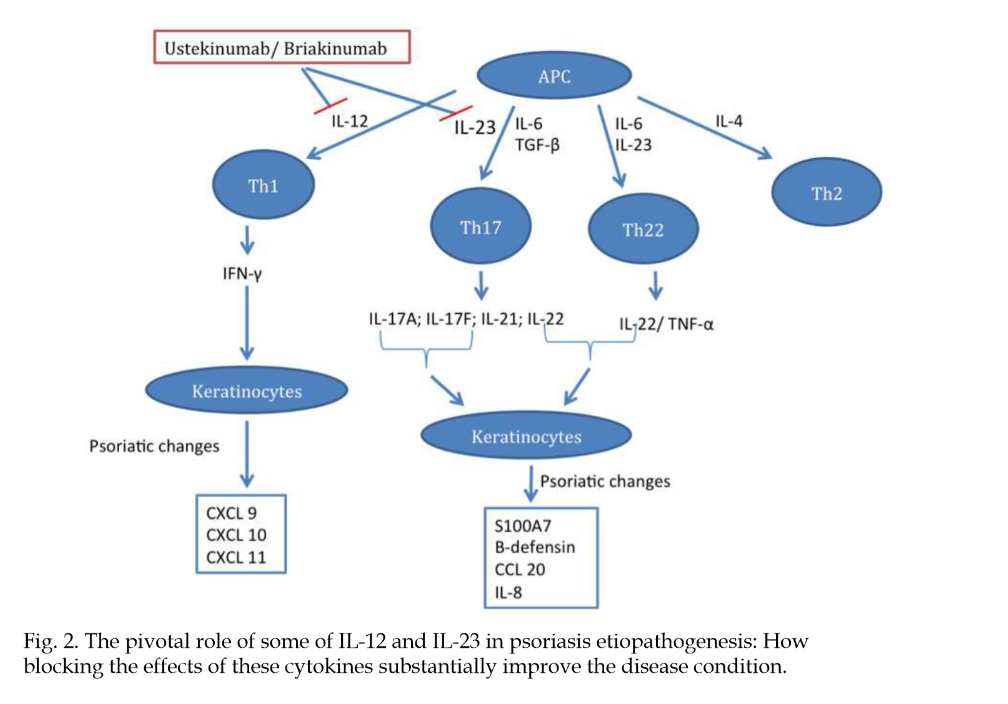
"An increase in efficacy and reduction of adverse events are the main drivers for new therapies. Infections, one type of adverse event, usually increase in patients receiving anti-cytokine therapy (Dinarello, 2003)."
"Some inflammatory agents, whether physical, chemical, or infectious, induce an intense immune response. This immune response against them frequently results in tissue damage that could be more intense if it were not for the interference of regulatory mechanisms (Belkaid et al., 2006). As has already been specified, Treg cells help limit the damage caused by a vigorous immune response."
"Similarly, excessive activity of Treg cells may limit the magnitude of the immune response, which may result in failure to control an infection. On the other hand, the absence of the T regulator may result in intense inflammation and autoimmune dermatitis. Tissue damage may also result from the development of effector cells against their own auto-antigens (Figure 4)."
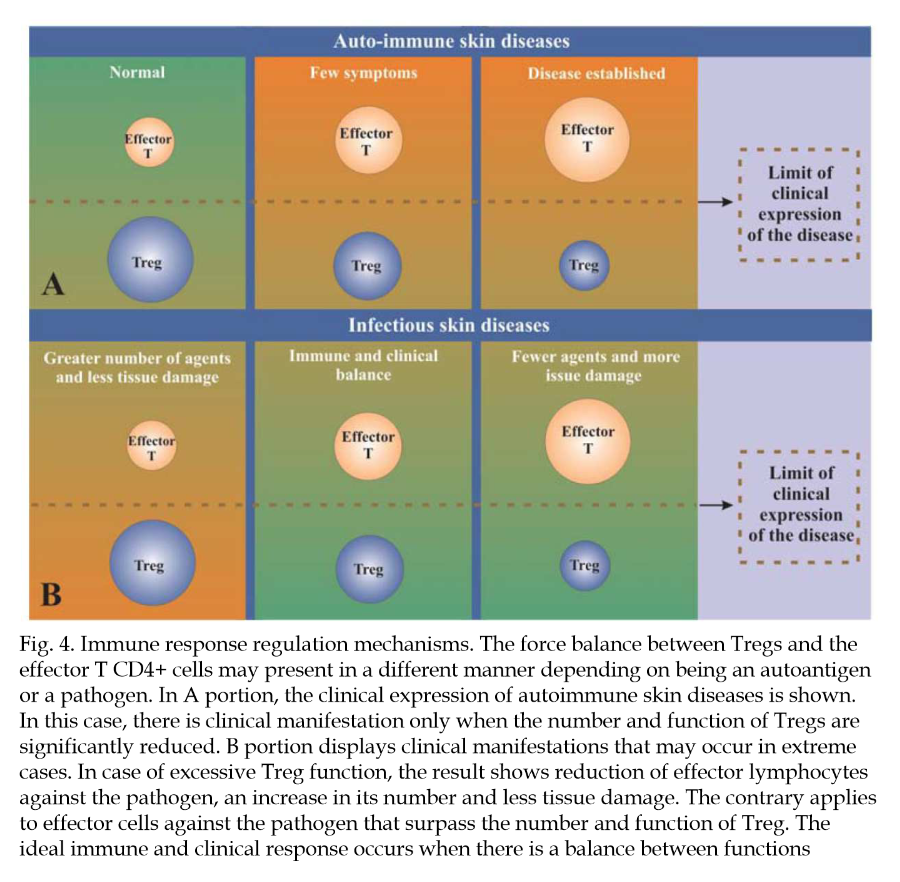
"An improved understanding of the role of T regulators in psoriasis may lead to the identification of new targets for treatment. More specifically, the goal is to manipulate natural regulator cells or those induced by means of an increase or decrease of their function, depending on the circumstances."
Immunopathogenesis of inflammatory bowel disease
"Altered gut permeability may lead to increased bacteria adherence and inappropriate exposure of the mucosal immune system to bacterial products causing inflammation."
"Negative regulation of the host innate immune responses to the indigenous microflora maintains gut homeostasis. Key players in the negative mucosal regulation include interleukin-10 (IL-10) and IL-2, as evidenced by mice with deficiencies in these factors develop spontaneous intestinal inflammation, but are protected from intestinal disease when raised in germ-free environments[77–>79]. This indicates that at steady state, pathologic consequences of immune activation by commensal microflora are constitutively inhibited by IL-10 and -2 dependent mechanisms."
"A subset of T cells producing IL-6 and IL-17, now known as Th17 cells, has emerged as an important mediator of the T-cell response in gut inflammation (Figure 4)."
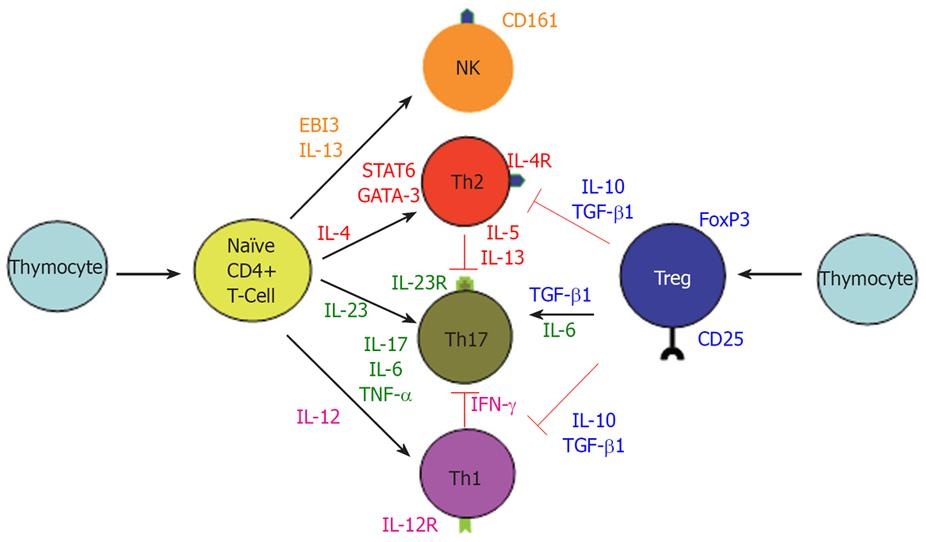
"The above findings do not exclude the role of exaggerated Th1 response in CD since IL-12/IFN-γ and IL-23/IL-17 may be parallel pathways involved in inflammatory response. Interestingly, IL-12/IFN-γ and IL-23/IL17 pathways are mutually exclusive, since IFN-γ suppress IL-17 and vice versa (Figure 4)[104]. It is of pathogenic importance to consider all of the major immune pathways that are responsible for the development of CD. For example, therapeutic targeting of only the newly discovered IL-23/IL-17 immune axis may actually exacerbate CD by accelerating the IL-2/IFN-γ Th1 pathway."
-
@Amazoniac
I hope others will contribute to this thread re absorption and clearance rates as well as mechanisms by which vit A may be poisonous/problematic. Are you personally limiting vit A intake at this time?I’m not (yet). Given what’s going on at RPF, I have, if anything upped eggs/milk/pumpkin/etc in my diet over the last few months to celebrate high-metabolic-rate good health, Ray Peat style, as quiet dissent. Like Amazoniac, I think it’s good for us to remain open to new information as it arises, however, about any potential problems associated with excessive vit A intake.
Might I get your advice based on my brief personal history, which was unusually high-dose vit A for several reasons? Growing up in the 1970s, my mom became a health-food nut and both mom and dad took – and gave me as a child – a large volume of vitamin supplements. From the age of 3 onwards (maybe through puberty), I was encouraged to take a daily beta-carotene supplement. We drank lots of carrot juice. The soles of my feet had an orange tint, which my doc may have even commented on once. We kept up this extraordinarily high diet + supplement regimen until I was a teenager. I took a round or two of Accutane for acne as a 17 year old. Under lots of other stressors and chronic sleep deprivation, I was diagnosed as a type-1 diabetic at age 20, which ran in my half-Norwegian family. I would be a likely candidate to have had – and very likely still have – excessive vit A stored in liver, adipose and elsewhere. Do you think I should try the low vit-A diet? (I can’t believe I’m actually writing those words…!!!...rest assured, only as a speculative hypothesis and not a likely action plan.)
-
@T-3
Adding: I never had (and don't think I have at present) any vit A toxicity symptoms. The callouses on my feet and palms are probably a bit more "orange tinted" than most people's and my complexion is noticeably darker than either of my parents, but I also get a lot of healthy sun exposure. My default is skepticism toward the hypothesis that I suffer from vit A toxicity. Eating high-vit-A foods makes me feel good and I don't think I have any problems associated with those foods. Thus, I'd say that worrying about vit A intake would be creating a pseudo diet problem where none in fact exists. But I gather that Amazoniac has made this thread because he has some concerns about vit A in his own diet, or is that wrong? -
https://www.bioenergetic.life/clips/8442a?t=813&c=17
"So a lot of these oils that are purported to be good for our health are actually quite thyroid toxic and long-term use could lead to conditions that are common in low thyroid and is as detrimental as cancer. Yeah. And there's one which isn't really a fatty acid, but it's a highly unsaturated molecule, carotene, which is the precursor to vitamin A. It not only blocks the cellular sites that use vitamin A, but as a polyunsaturated molecule, it also blocks the thyroid function every place that the vegetable oils do."
-
@DavidPS Thanks for posting this quote from Ray. Apropos! DavidPS, are you limiting eggs, oranges, persimmons, pumpkin, sweet potato and the like?
Is the retinol in milk different from the beta carotene in orange colored foods?
I think I'm asking embarrassingly basic questions here. And I worry my advice request may inadvertently be derailing what Amazoniac wanted this thread to be about. I just wanted to get expert advice re practical take-aways from those of you thinking carefully about the evidence base from published studies regarding risks of excessive retinol and/or beta-carotene-containing foods. Has what you've learned about vit-A risk changed the way you eat?
-
Most here are familiar with RP's discovery that a daily carrot salad made him feel better. Some think it was because of the "colon sweeping" effect, but if that were the case, wouldn't charcoal have the same effect? Could it be because Beta carotene is an effective modulator of the gut biome?
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10220829/