Adamantyl Ester Steroids: The Ultimate Androgen?
-
@alfredoolivas wtf i was told deca was better…maybe its cause its testing these two muscles and the other reference was on other muscles or even humans idk
-
@sushi_is_cringe yes there likely exists far better evidence to answer this question
-
@alfredoolivas adamantyl steroids are less hepatotoxic and have fewer sides than most esters, which could let you dose higher safely. So even if the release is slower, you might still get more total exposure over time. I’m also scratching my head at the study, how can the half-life of adamantyl be shorter compared to other steroids when we know the adamantane ring is so stubborn to metabolize on its own? A 2012 study in Steroids (Vol. 77, Issue 5) noted that “adamantane derivatives exhibit remarkable stability against enzymatic degradation, often extending half-lives significantly” (p. 419). Maybe the dosing or assay setup skewed things here?
-
@davedavidson Half life = release. The longer the half life, the longer the release. The study showed that the adamantane ester steroids had a shorter half life and were released faster.
The ester of a drug isn’t metabolised - it is cleaved off the drug. Admantane was simply cleaved off the steroids, regardless of its half life. It wasn’t metabolised.
Propioante and other carboxylic acids have a half life of a few minutes when injected as they are metabolised into energy - yet when they are used an ester for steroids it can make their half life up to a month long. So your argument for metabolism resistant = longer half life doesn’t make sense, as the ester isn’t metabolised it is simply cleaved off.
Whilst it’s true that adamantane hasn’t got an enzyme to degrade it into metabolites, it can still get urinated out of the body - , the adamantane derivative amantidine has a half life of 12 hours.
-
@alfredoolivas do you think changing the position of the ester to 3a or 3b cause it to cleave slower? Or maybe a double ester
-
Why would you put an ester on 3alpha? And what exactly would this accomplish? If you want to use illegal steroids to make gains, there are much better and proven to be effective ways of doing this, such as using drostanolone.
Besides that, do you have any idea how difficult it is to do organic chemistry like this? You would need a lab, advanced knowledge with a university degree most likely, and provable ways to demonstrate both the effectiveness of the synthesis and the absence of contaminants in the finished product.
It seems like based on the OP that you're thinking about taking this orally @davedavidson Why? How do you know its orally bioavailable?
Think about what this this 3 alpha derivative would entail. It would entail soliciting the development of a novel (and weird) derivative of androsterone or DHT to be taken orally (when there is no evidence that its orally bioavailable) for what reason exactly?
I feel people are really desperate to reinvent the wheel for no good reason. If you want to use illegal steroids to make gains that are known to be safe (relatively), use Masteron.
-
3 alpha is where the ketone functional group for DHT is - which is what allows it to be an androgen. So, if you know anything about organic chemistry you would not mess with that if you want the resulting compound to behave like DHT in the body.
-
After looking more into the user's suggestion of putting an ester on position 3 alpha, there is actually zero reason to think it would do anything at all.
That is because 3 alpha is where the ketone is for DHT, and the hydroxyl group is for androsterone, respectively.
@davedavidson you cannot simply attach moieties to the position at which the functional groups of the steroid is and expect the resulting compound to be anything but a clusterfuck of a compound that is biologically inert.
That is why oral steroids have a methyl group at position 17 alpha. Because position 17 beta is where the hydroxyl group is. They do not mess with 17 beta.
BTW: @alfredoolivas masteron is actually 2alpha-methyl not 3-alpha methyl. As far as I am concerned @davedavidson is the first person to ever think of putting an "ester" of position 3 of an AAS

-
@jamezb46 I mean 2 methyl DHT!
-
Putting aside the proposal to insert adamantane at 3α, what does everyone think of @davedavidson's claim that oral androsterone (or DHT) is "torching the liver"?
Respecting that people's responses to androsterone seem to vary markedly, I share @davedavidson's appreciation for androsterone and DHT's psychological effects.
I've seen the hepatoxicity concerns raised numerous times about oral androsterone on RPF/LTF (e.g. on the androsterone mega thread). What does everyone think about oral androsterone at 4 to 8 drops daily? Would that be "torching the liver"?
Have we seen studies documenting such problems at these (or higher) doses?
-
@jamezb46 you are drilling into him a bit too hard haha. He just innocently suggested something impossible haha
@davedavidson as James said ketones can’t get esterfied. Only hydroxyl (OH) groups can be esterfied.
Since most androgenic steroids have a ketone group at position 3, they can’t be esterfied at position 3. For example, androstenedione that has no hydroxyl group and only ketone groups at position 3 & 17 cannot be esterfied.
However @davedavidson, Androsterone, DHEA and pregnenolone have a hydroxyl group at position 3, so there can be an ester at position 3 of these steroids. Pregnenolone and DHEA acetate have the ester at these positions.
But in the end as James said… we are absolutely spoiled with choice for steroids, that are pure and dirt cheap and they work profoundly well.
We already have androgenic, anti estrogenic, progestogenic, anti mineralocorticoid, GABAergic and anti cortisol steroids all you can buy with ease online for a low price.
So just take your test and shut up:)
-
@T-3 that’s BS. Only steroids with added functional groups to the outside of the steroid skeleton “scorch” the liver, and they do so to so many varying degrees.
Androsterone, DHT, testosterone, have no functional groups artificially added to the outside skeleton of the steroid, and are perfectly safe for the liver. Doses of 600mg testosterone undecanoate orally daily (70% bioavailability) have been shown to have no effect on the liver in a clinical trial. Injected testosterone has shown to cure liver disease.
However, when you add functional groups, they tend to increase liver enzymes. For example, 2a-Methy DHT is DHT with a methyl group added to it and it’s known to slightly increase liver enzymes whereas DHT isn’t.
However certain functional groups in certain positions have different levels of harshness on the liver.
-
Generally, the more orally bioavailable a steroid is, the more hepatotoxic it is.
The hepatotoxicity is a measure of how resistant the steroid is to the liver’s attempts to metabolize it.
Why does anyone think that 4-8mg of androsterone is hepatotoxic? What’s stopping the liver from attaching a sulfate group to it to make androsterone sulfate?
-
Oof okay, DHT’s 3-keto can’t be esterified without reducing it, and thatd just tank its activity, I kno im retarded but the responses here are just dogmatic lecturing “take your test and shut up.” It’s condescending and kills any real discussion. I’m not trying to just follow the same old path everyone’s been on since the 50s.where’s the progress?? if you’re not open to exploring new ideas why even engage??
-
@davedavidson it was a joke, sorry I thought the “:)”made it obvious.
You aren’t retarded at all, I didn’t paint you out to seem that way. I just thought you were a little to optimistic about a steroid that was popularised by Haidut, the master of conjecture.
It's quite personal to me because I too went down the rabbit hole of listening to Haidut's conjecture... buying all the overpriced Idea Labs products, applying hundreds of milligrams of DHT and pregnenolone on my body to create the optimal "anti-catabolic steroid", listening to his weight loss advice etc... it left me with a massive hole in my wallet and fatter.
This is why I ravantly encourage people to present their own evidence, and not follow what Haidut said 10 years ago on a forum or what the X echo chamber is saying, as it is a lot of the time they are spreading info that is 100% conjecture and is a waste of time and resources. Being serious.
-
I thinks it's an interesting thought. But I don't really see the upside here. It mainly appeals to people because it's mysterious and hard to obtain etc.
I get it... the first email I send Ray was about an obscure progesterone/DHT mix of a designer steroid , which I'm surprised he even replied to
But if you want DHT with a longer half life you can use 11ketoDHT.I still think 11ketoT is an interesting option on paper. IIRC I saw a study suggesting it doesn't even aromatize.
-
@T-3 said in Adamantyl Ester Steroids: The Ultimate Androgen?:
I've seen the hepatoxicity concerns raised numerous times about oral androsterone on RPF/LTF (e.g. on the androsterone mega thread). What does everyone think about oral androsterone at 4 to 8 drops daily? Would that be "torching the liver"?
Have you looked for studies on that ?
Androsterone is an FXR agonist, which are used to treat liver disease. So if anything it should help the liver.
-
@Mauritio 11-keto steroids are extremley interesting - 11-keto testosterone can get reduced back into 11-hydroxybeta-testosterone, which is very similar structurally to cortisol - it has the same double bond on position 3-4, the 3 keto group and the 11 hydroxl group.
11 keto progesterone looks insane; it turns back into 11-hydroxybeta progesterone which is basically corticosterone without the OH at the top.
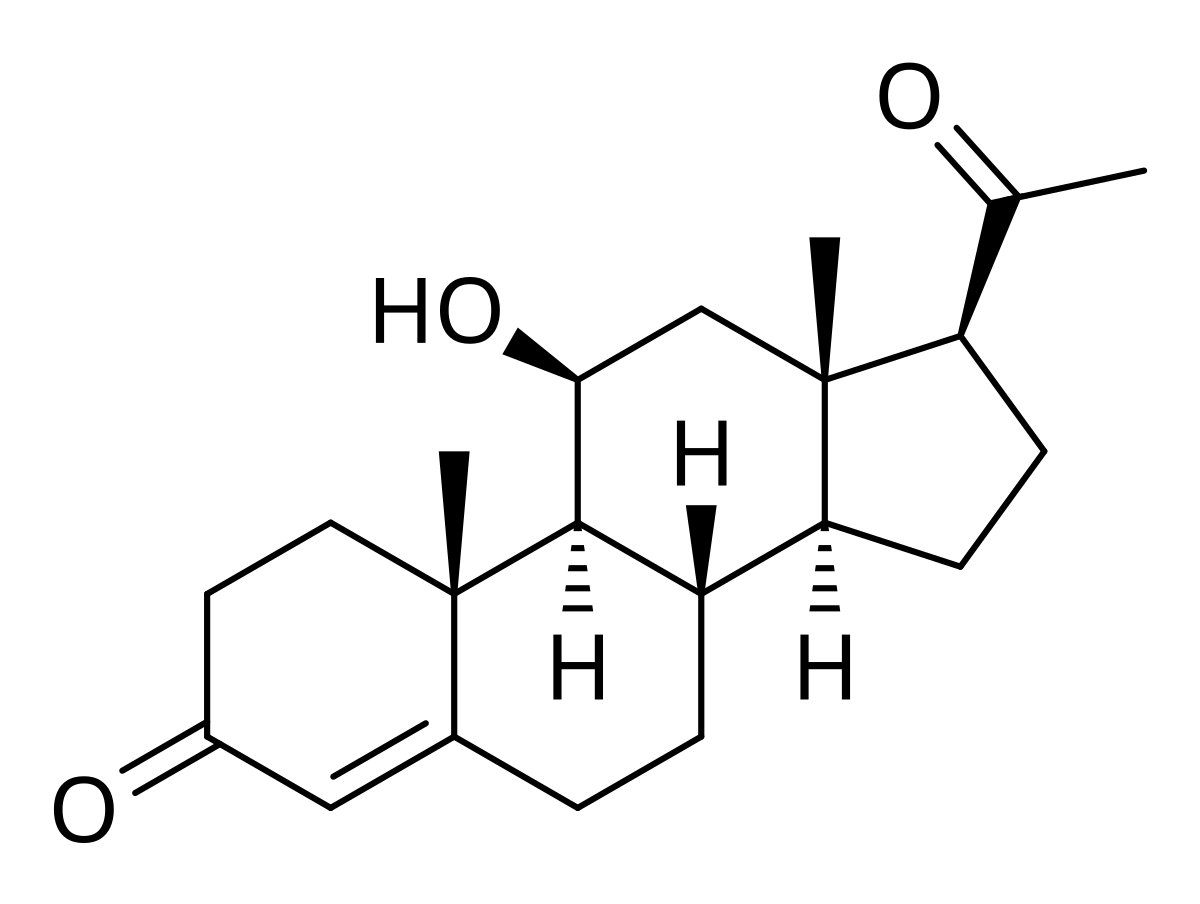
11 betahydroxy progesterone
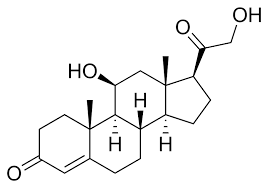
corticosterone.This structural similarity will make it into an insane anti-glucocorticoid. Progesterone binds to the GR receptor with around 8% the binding affinity as cortisol.
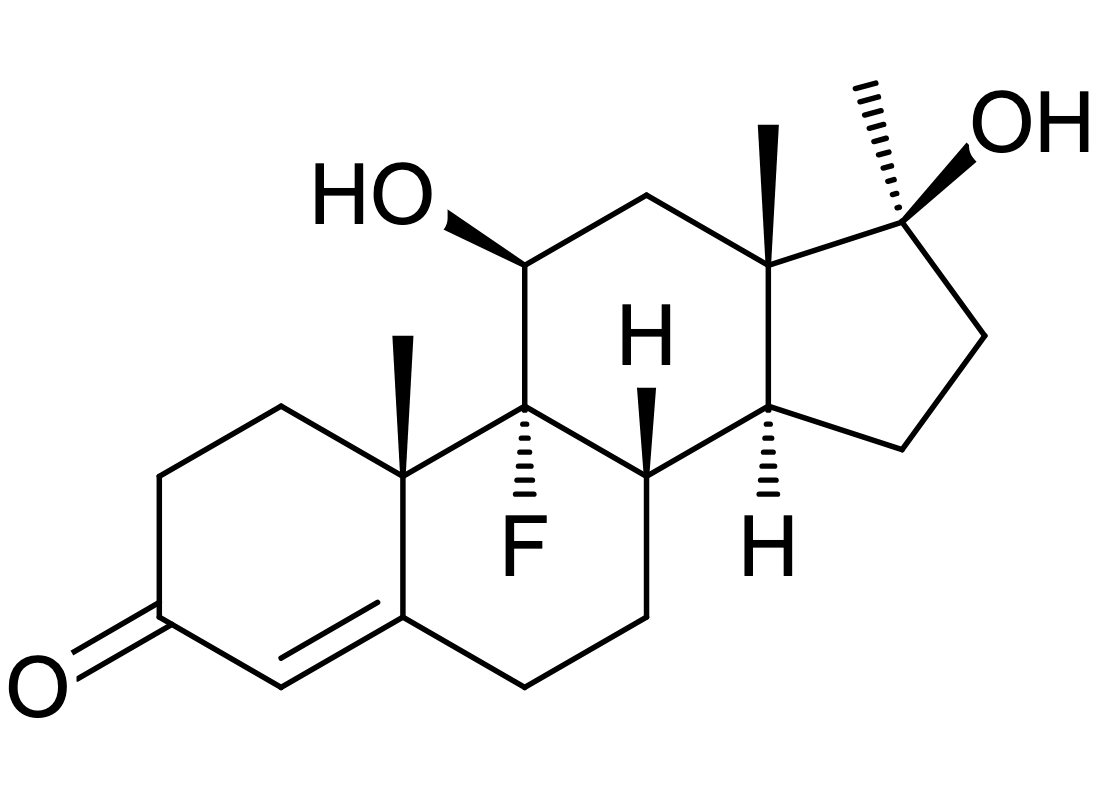
it is known that the addition of a hydroxyl group at position 11, increases binding to the GR receptor. Fluoxymesterone has a hydroxyl group at position 11, and it binds to the GR stronger than testosterone.
It is structurally similar to dexamethesone
fluoxymesterone
dexamethesone
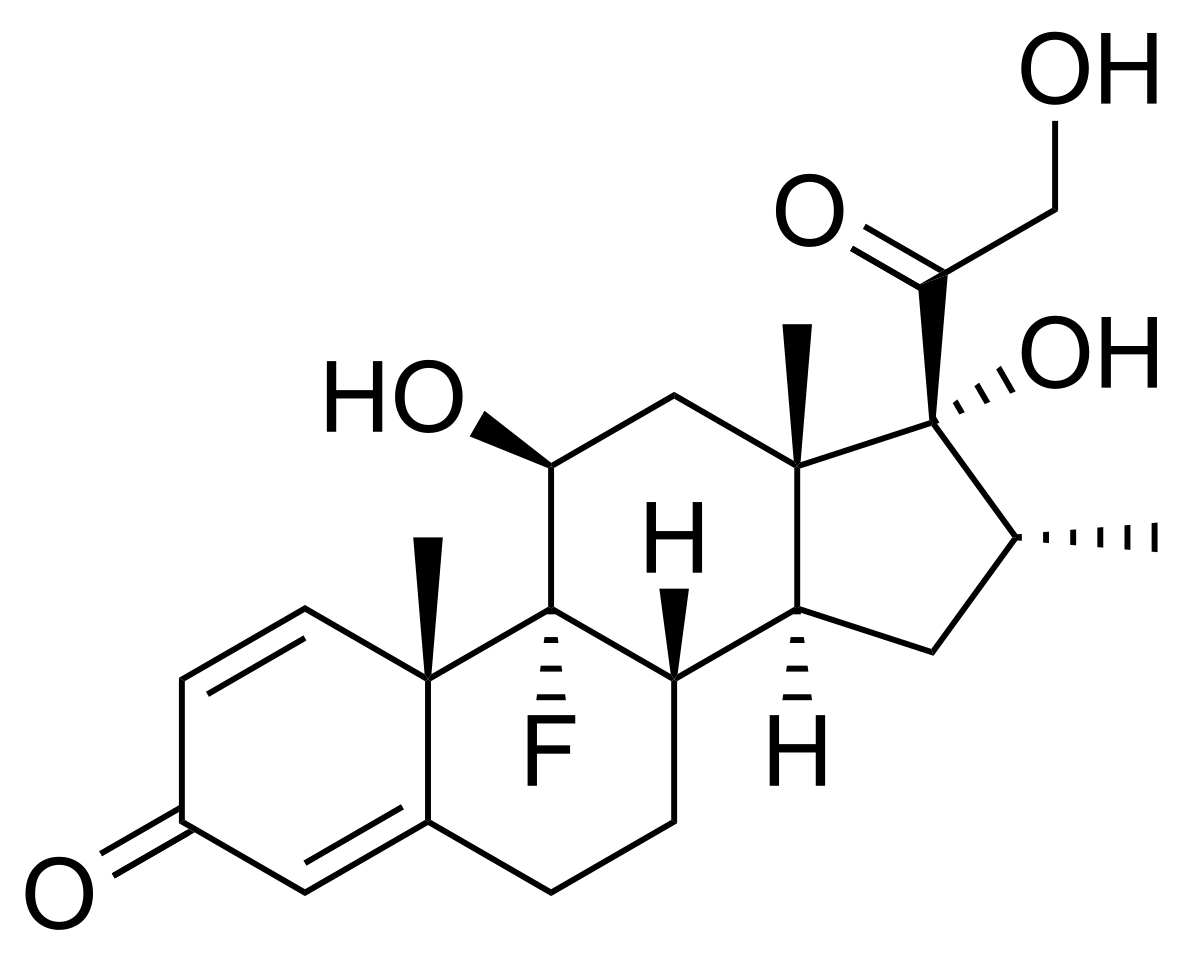
So 11 beta hydroxyprogesterone will probably be an even more strogner GR antagonist than progesterone is - 8% relative to cortisol is very high already.
-
Patrick arnold is still selling 11-KT.
Pretty expensive, but here it is
https://prototypenutrition.com/collections/all-products/products/11-kt-spray-original-formula
-
@jamezb46 Really good doses, 250mg of 11-KT per dose, using alcohol based solvents! It's a massive bottle and a single dose is 50 sprays, it isn't clear how many doses are in a bottle though.