@Ibshaver23 Are you male or female? If male, you could try taking 100-200 mg pregnenalone instead, or look into anti-inflammatory peptides like BPC-157 or KPV
Latest posts made by jamezb46
-
RE: Sciatica & progesterone dependencyposted in Case Studies
-
Knowledge is Powerposted in Philosophy
I feel as if people greatly misunderstand this Nietzschian concept.
It is not a positive thing; it is not a triumph; it is not something to be celebrated.
At best, it is a special case of Nietzsche's thesis that everything is subordinate to the will to power, including knowledge.
If true, it means that truth is dead. It means that our understanding, like a bird with broken wings, tries to fly toward truth but crashes to the earth.
Knowledge is power is not something to be happy about. It is a tragedy.
-
RE: B vitamins are dangerous?posted in Not Medical Advice
What does your body composition and weight look like and what is your BMI? How long have you been depleting PUFA? How are your BMs? Do you eat organ meats?
-
Anavar and Caffeine, revisitedposted in Literature Review
Many of you (you know who you are) either are or should be aware of the fascinating Portuguese study on how caffeine affects oral oxandrolone concentration.
https://www.adop.pt/media/4114/Oxandrolone_excretion_effect_of_caffeine_dosing_.pdf
The basic design of the study was as follows: A 400 microgram (0.4mg) dose of oxandrolone was administered to a "regular drinker of caffeine", reported to be equivalent to around 3 espressos a day. Urine was collected for 70 hours following.
4 days subsequent to the initial administration, 400 micrograms of the same was co-administered with 300 mg caffeine.
The results are displayed below:
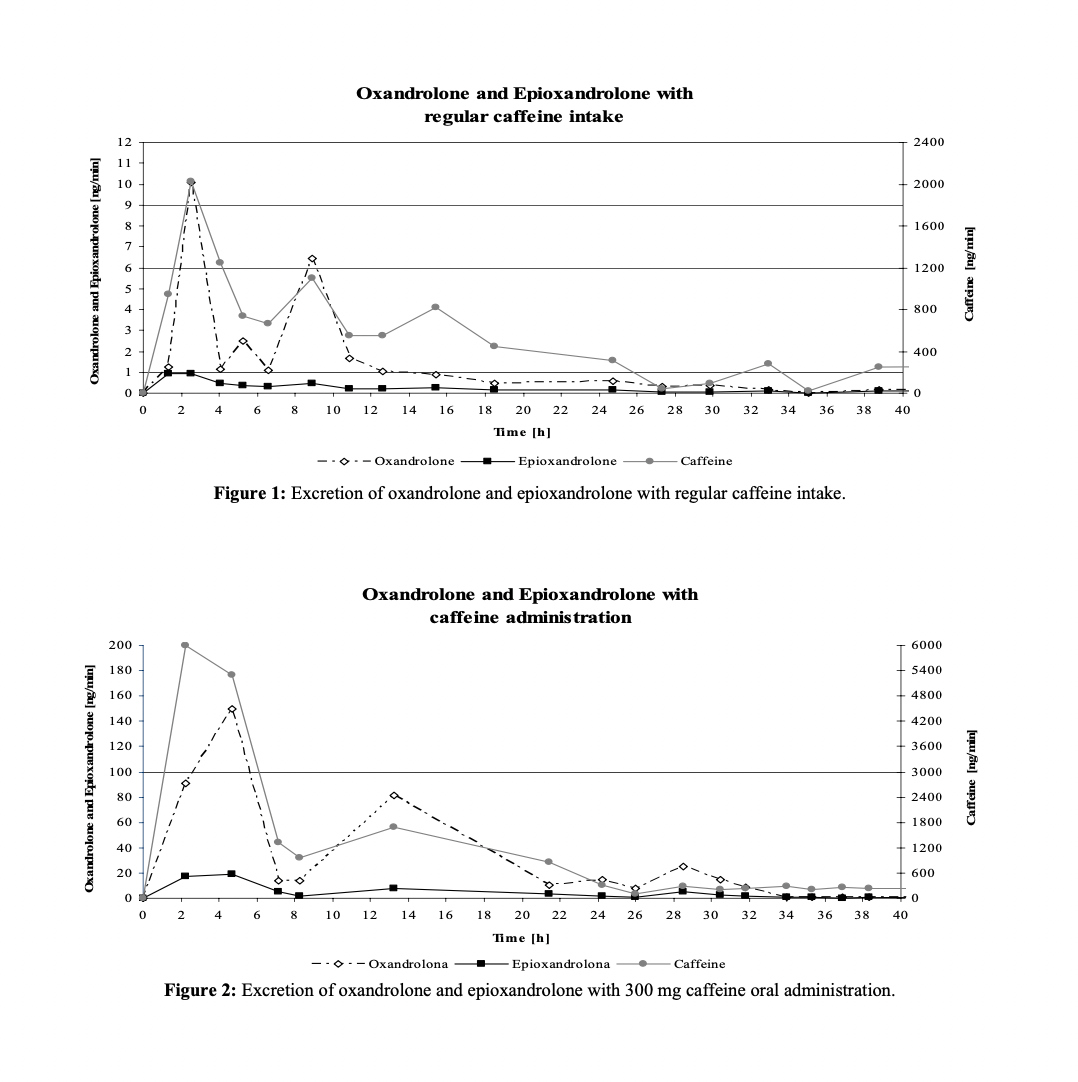
Clearly, when caffeine was co-administered with oxandrolone, the rate of excretion (measured in ng/min of urine) of oxandrolone was significantly greater than that when caffeine was not co-administered.
The quantity represented, however, is perhaps not as informative as we would like. After all, ng/min is a bit of a strange unit.
Ideally, we would also have the total amount of oxandrolone excreted under the two conditions
While the study authors did not provide such a figure, I estimated it by hand from the above data using trapezoidal approximation.
The total micrograms of oxandrolone excreted without caffeine was approx. 2.8 mcg, or about 0.7% of the oral dose by weight.
The total micrograms excreted with caffeine was approximately 90 micrograms, or about 22% of the oral dose by weight.
Now, the study also measured the amount of the major metabolite of oxandrolone, epioxandrolone.
Oxandrolone is only negligibly metabolized to epioxandrolone, however, so I did not calculate the AUC for that metabolite.
The study authors conclude the study by saying that, not only did the maximum concentration of oxandrolone in the urine for caffeine+oxandrolone almost 20x the maximum in the oxandrolone only trial, but max amount of epioxandrolone was only 15x that for oxandrolone solo.
Therefore, in the caffeine + oxandrolone trial, not only was far, far more oxandrolone excreted, proportionally less of what was excreteted was epioxandrolone
These remarkable findings raise the question: how do we explain these results, and what are their implications?
Well, the fact that there was approximately 30 times the amount of oxandrolone excreted when it was administered with caffeine suggests that caffeine massively increased the absorption of oxandrolone.
And indeed, caffeine is a known inhibitor of P-glycoprotein transporter, which normally causes oxandrolone to be retained in the gut and not absorbed.
The 30x increase, however, is puzzling. It seems unlikely that PGP alone could explain that
Perhaps the decreased proportion of epioxandrolone produced can be explained by caffeine inhibiting CPY enzymes slightly.
We need, however, a satisfying explanation for why this 30x increase was observed
It could not be that oxandrolone was somehow retained more in the body when it was administered alone. That is not how oral steroid metabolism works. We should assume that the difference in amount excreted is explained by a difference in the amount absorbed.
Why this is the case, I am not completely sure.
-
RE: Patrick Arnold's Prohormonesposted in The Gym
The point of this post is not to propose some new fancy end-point androgen.
We already have highly effective AAS, and we don't need new ones.
The point of this post is to point out that, despite appearances to the contrary, the door to prohormones sold as legal, dietary supplements IS NOT closed.
The 11-keto-4-androstenediol should be legal and highly effective as an oral precursor to 11-KT.
THe key is in the marketing. If it is marketed as "anti-cortisol" "adrenal support" it should not be declared illegal by FDA.
The additional anabolic control act laws passed in the early 2000's have to do with analogues to androgens that are marketed for muscle building.
-
RE: Patrick Arnold's Prohormonesposted in The Gym
It's my understanding that regular Ursolic acid would not exert any effects. Perhaps it can disrupt bacteria in the gut but I don't think it's getting absorbed.
I have not tried any of his products yet. If I had to go with one I would actually go with the D-Serine for the mental effects.
Like I indicated in the OP, I think that 11-Keto-4-Androstenediol would be a highly effective oral prohormone.
The only problem is that it doesn't exist. It is found in tiny amounts in animals but AFAIK no one has synthesized it into a supplement.
But for the reasons I presented, it should be a quite efficient precursor to 11-KT orally in humans.
-
RE: Patrick Arnold's Prohormonesposted in The Gym
The problem with the reasoning that 4mg of oral ursolic acid is effective is that ursolic acid is not bioavailable. It doesn’t solubilize in stomach acid and it isn’t readily absorbed.
If the 4mg per day was calculated from an in-vitro study, that represents a fatal flaw. The issue with ursolic acid is absorption. That is why petri dish studies may not translate to equivalent oral doses.
The Ursolic acid in the Ur-spray product is Arginine Ursolic Acetate. That is a synthetic derivative that Arnold invented as far as I know.
No other company I am aware of sells it. It is soluble and thus will absorb through the skin.
Keep in mind I am no Patrick Arnold shill, but with that product specifically, there is some nuance and it is known that standard ursolic acid does not absorb transdermally or orally.
-
Patrick Arnold's Prohormonesposted in The Gym
TLDR; A new and very effective test booster probably exists, it just needs to be synthesized
Background
For those who are not aware, Patrick Arnold is a synthetic organic chemist most well known for his involvement in the BALCO scandal that involved the distribution of "undetectable" steroids through Victor Conte's network to several high profile athletes.
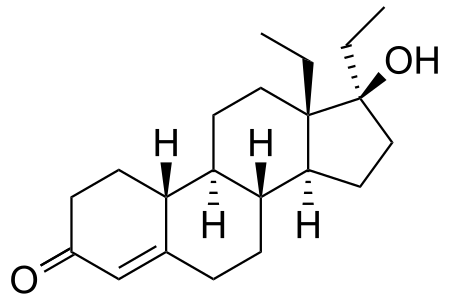
The first undetectable steroid, was dubbed "The Clear" because when people who were taking it were internally tested, their bloodwork would come back "clear". It was also known as Norbolethone. Structurally, it is nandrolone with an ethyl and methyl group on carbon 17 and 18, respectively. It was an abandoned synthetic steroid from earlier in the 20th century.
Norbolethone
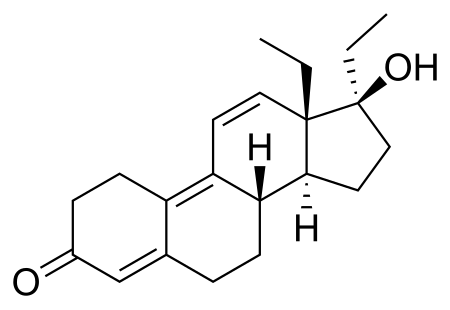
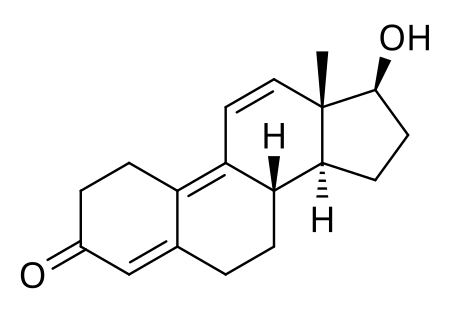
The second and most famous, was Tetrahydrogestrinone (THG). It was Arnold's original invention. He was the first man in history to have synthesized it. It was called "The Clear" as well, but was also known in Arnold's inner circle as "Tren stuff". Structurally, it differs from Trenbolone only by an ethyl and a methyl group, on carbon 17 and 18, respectively.
THG
Trenbolone
Prohormones
In addition to his fame from developing undetectable steroids, Arnold also developed several prohormones available as nutritional supplements from the now extinct company ErgoPharm.
The concept of a prohormone is a substance that the body converts to an active hormone. The prohormone has little biological activity of the kind that the active hormone has. In that sense, the body transforms the prohormone into a much more active compound. In Arnold's case, he wanted the active hormone to be an AAS.
The prohormones that Arnold developed followed a similar developmental progression as his Clear drugs.
The first prohormone he considered was orally administered Androstenedione.
Androstenedione is a so-called one step precursor to testosterone because the action of a single enzyme is sufficient to transform it into testosterone. As the -one suffix suggests, androstenedione has ketone groups on position 3 and 17, while testosterone has a ketone on 3 and hydroxyl on 17. Therefore, 17B-HSD converts androstenedione to testosterone. The conversion rate is approximately 6% in man (https://web.archive.org/web/20110318185140/http://www.patentstorm.us/patents/5880117/description.html)
Naturally, that is pretty disappointing. Therefore, Arnold investigated 4-androstenediol (4-AD),
which is naturally present in humans. This molecule is also identical to testosterone, except for a hydroxyl group on position 3, which must be oxidized by 3B-HSD. Unlike Androstenedione, 4-Androstenediol converts at 15.6% to testosterone when given orally.
In fact, after given at a dose of 100mg orally, subjects experienced a 48% increase in total and 43% increase in free testosterone 90 minutes post ingestion.(https://web.archive.org/web/20110318185140/http://www.patentstorm.us/patents/5880117/description.html)
Obviously, 4-androstenediol is about three times as effective as androstenedione. Arnold, however, took things even further with the even more potent 1-androstenediol.
1-androstenediol (1-AD) is also naturally occurring, but in tiny amounts. It has the same hydroxyl structure as 4-androstenediol, but a different ring structure. Instead of having an unsaturation from carbon 4 to 5, it has one from 1 to 2. This anomaly is also seen in powerful AAS like Boldenone,Dianabol and Turinabol. The 1,2 delta bond makes the androgen much more anabolic.
It is pretty clear that Arnold gained the knowledge that the 3B-HSD is much more efficient than 17B-HSD when acting on orally administered androgen analogues, hence his pursuit of 1-AD and not 1-androstenedione. Oxidation at position 3 creates 1-testosterone AKA Dihydroboldenone, which is a very powerful androgen.
1-AD was known across the industry when it was sold by E-pharm as very powerful, similar to oral steroids.
Unfortunately, the FDA banned all of his prohormones. They are no longer available at all. To my knowledge, not even underground peptide websites or steroid dealers have them simply because no one synthesizes them anymore.
Currently Available Products
Arnold is still around, and makes supplements for Prototype Nutrition.
Many of the supplements on his site have excellent research behind them, including D-Serine and DHEA. He posts research supporting the use of the supplements on the product pages.
Among the most interesting anabolic products he sells are an 11-Ketotestosterone (11KT) spray and an Adrenosterone(11-OXO) oral suspension. Notably, Adrenosterone (11-OXO) has the same relation to 11KT as androstenedione has to testosterone.
11-OXO

11-KT
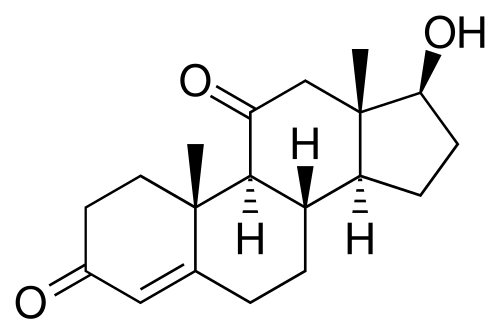
That is to say, 11-OXO is converted to 11KT by 17B-HSD. If we assume 17B-HSD can convert the former to the latter with the same efficiency as it can convert androstenedione to testosterone, a 100mg oral dose of 11-OXO would equate to 100/20=5 mg of 11KT. 11KT is the dominant circulating androgen in fish, and is known to possess biological activity in human males comparable to testosterone.
5 mg of a testosterone equivalent circulating around the body is significant, and anecdotal reports suggest that indeed 11-OXO is both non-suppressive and powerful in aiding fat loss, which makes sense given that it can reduce 11B-HSD type I expression.
Somewhat remarkably, it also turns out that 11-KT is the dominant androgen during Adrenarche in humans, which is a period that extends from about 2 years prior to puberty to about 20 years of age in healthy populations. It is therefore highly likely that it is a powerful libido booster. (https://pubmed.ncbi.nlm.nih.gov/30137510/)
If users want to try experimenting with something, then 11-OXO or 11KT could be a good option. Perhaps people will have excellent responses, and we can pressure IdeaLabs into creating their own product.
Future Development
Given that the FDA permits the sale of 11-OXO, then we can take Arnold's previous research to once again develop what should be a legal and powerful prohormone.
What should the chemical structure be? Well for those who have been paying attention, it would replace the ketone groups of 11-OXO with hydroxyl groups, thus allowing for its conversion to 11-KT via the more efficient 3B-HSD pathway.
I think it would be called 11-keto 4-androstenediol.
-
RE: Lack of brain glucose in high stress momentsposted in Not Medical Advice
@lobotomize-me If you need to become numb then you can try propranolol