The future of oral testosterone
-
@alfredoolivas That is NOT 4-androstenediol. That is 4-DHEA, which is a prohormone to 4-androstenediol. No one dares sell 4-androstenediol on the open market in the U.S after what happened in the early 00's. But my point is that UGLs don't give a damn about the law anyway, so if enough people ask for it, they should be able to sell the real thing (not the B.S 2 step precursor)
-
@jamezb46 said in The future of oral testosterone:
As you can see, the TU in arachis oil only spiked Testosterone in the blood highly when it was given with a fatty breakfast. Otherwise (in the fasting participants) it did not perform better than base testosterone.
Testosterone Undecanoate in Arachis Oil has a 40% bioavailability, and it increased tesostereone by 10 n/mol.
It does spike testosterone. However, when you compare it to the levels when eaten with a fatty meal? Sure it spikes it less.
But it does spike testosterone effectively... as shown with the 40% bioavailability.
Furthermore, a large proportion of people eat a fatty breakfast. So if you eat a fatty breakfast? You can expect 100% bioavailability. So testosterone undecanoate seems like an excellent option, especially for those that eat a high fat diet, which many Americans do.
@jamezb46 said in The future of oral testosterone:
To be clear, using 600 mg free test base per day is dirty cheap. It is about 50 cents per day!
If you buy 100g of TU from Purple Panda Labs, each gram costs 1.14 dollars.
600mg each day would cost 68 cents a day.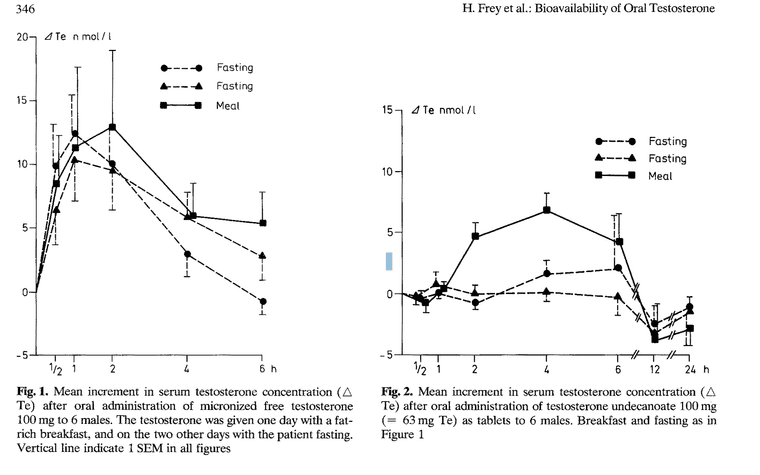
TU has a much longer half life than Test base, so thats why I reccomend it
-
@alfredoolivas Fair point on the TU. I concede that it should work too.
But, the long half life is a double edged sword. What if I specifically want a big peak in the morning and pre workout so that overnight I can recover from any suppression?
For me, though, I would want to just go with the base hormone (testosterone) due to weird interactions that may happen with the esterified hormone not being recognized by the body and because if I want to dose it multiple times per day, I may not do it with a fatty meal (like pre-workout)
What I really want, however, is for 1-A-DIOL (1-AD) to come back to the market.
-
@jamezb46 Does 1-AD turn into 1-testosterone and does it turn into to 1-dihydrotestosterone (dihydroboldenoe)? Any reason why the double bond on carbon 1 is better than carbon 4?
Glucocorticoids thhat have the added unsaturation between carbon 1 and 2, bind to higher affinities to the GR, but androstane steroids have a very low affinity for the GR, regardless of their unsaturation; they exert their anti-catabolic effects via genomic changes from activating the AR. So I assume thats not why you want the unsaturation of carbon 1.
-
@jamezb46 btw, I finished by bottle of tren. I don't see anything very special about it, after running 100mg a week subcutaneously for a month. I don't think I will be buying it again. Apart from turinabol, are there any other (available) AAS I should look into or perhaps you are interested in?
-
It turns into 1-testosterone, which is DHB. The androgen with the normal 4,5 delta bond AND the 1,2 delta bond is boldenone.
Steroids with the 1-2 double bond are thought to be more anabolic than steroids without the 1-2 double bond. I don't know if that is because they bind better to the AR or if they are more anti-catabolic by stopping glucocorticoid induced catabolism or if they work through some other means.
-
@jamezb46 very interesting - it can turn into dihydroboldenone without being 5 alpha reduced ?
-
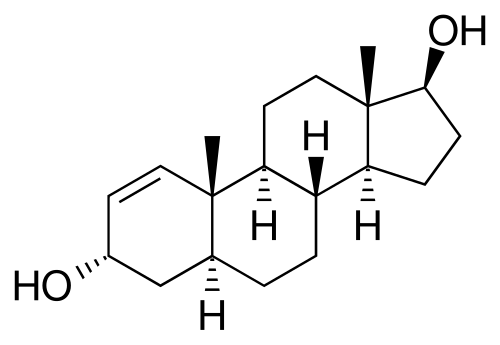
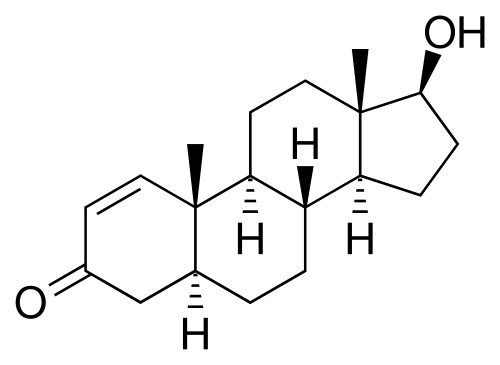
1-AD is saturated at the 4-5 position so it is already "5 alpha reduced". All that it needs to turn into DHB is for the alcohol on position 3 beta to be oxidized to a ketone.
1-AD
DHB
-
@jamezb46 Very interesting, thank you! I am learning a lot; 5 alpha reduction is the simple removal of the carbon 4 double bond; the addition of the extra hydrogen that is bonded to the carbon, simply occurs because the carbon isn't sharing 2 electrons to the other carbon atom anymore, so a hydrogen shares its electrons to give the carbon a full valence shell.
So a dihydro-steroid is simply a steroid that is missing it's double bond on carbon 4 and therefore has an extra hydrogen. I guess the conversion of 1-AD to 1-testosterone is exactly the same as the conversion 3alpha androstanediol into dihydrotestosterone.
-
Almost the same. Technically 3 alpha androstanediol (if it ever is converted back to DHT) would have to do so through 3 alpha HSD. But 3 alpha HSD is not very active as an oxidant, it usually only reduces DHT to 3 alpha androstanediol.
Since 1-androstenediol has the hydroxyl on 3 at the beta position, 3 beta-HSD coverts it to DHB, and that happens much more readily.
BTW: Since you asked about compounds to try, I think 11-keto testosterone should be interesting. It cannot aromatize, is equipotent to testosterone as an androgen, and has anti-cortisol effects due to the ketone on 11. Sounds like winstrol w/o the liver tox
-
In this video, Haidut claims (in technical details) that mixing androgens with saturated fats like ghee or egg yolks helps a lot with effective absorption https://youtu.be/lfdNtcc0P4I
-
Thanks for the link. Egg yolks may have a unique mechanism because of their phospholipids. So, perhaps mixing micronized testosterone with egg yolks would help with absorption.
However, in the study @alfredoolivas posted, it seemed that blood levels of testosterone after oral administration of free crystals at 100mg were largely unaffected by a high-fat breakfast.
That is to say, blood levels of testosterone following a 100 mg oral dose of testosterone as free crystals rose just as high when fasting as they did after a high fat meal.
-
Sounds like any amount raising your total Testosterone to more than 4 milligrams a day is likely to turn into estrogen:
Post in thread 'Ray Peat Email Advice Depository'
https://lowtoxinforum.com/threads/ray-peat-email-advice-depository.1035/post-386792…
Ray Peat said:
… A healthy young man produces about 4 mg of testosterone and 12 to 15 mg of DHEA per day, so 80 mg per week [of testosterone] seems hugely excessive. An excess of either is likely to be turned into estrogen. Pure pregnenolone would be better, but I don’t know of a current product that I would trust to be free of estrogens. HCG isn’t safe." -
This post is deleted! -
this??????
https://www.predatornutrition.com/prohormones/fusion-supplements/androtest.html

also, it's in cyclodextrins so will absorb very well
-
https://forums.steroid.com/showthread.php?t=244033&p=2760655#post2760655
"Progesterone for Men
Once again, I am not crazy. I know that most of you think of progesterone as an "evil" catabolic and fattening hormone. What many of you may not remember is that the extremely popular anabolic steroid nandrolone decanoate (aka Deca Durabolin or nortestosterone) is in fact classified as a progestin (hormone with progesterone-like activity)! In addition, many progestins given to women in birth control pills and other drugs such as norgestrel and norethidrone are classified as 19-nor-testosterone or 19 nor- progesterone derivatives. Eastern German female Olympic athletes were known to have taken large quantities of these nor-testosterone derivatives to build muscle with the notorious masculinizing side effects that was obvious to all Olympic observers. Modern Olympic testing can now distinguish the difference between nor-progesterone and nor-testosterone derivatives. Since birth control pills aren’t yet on the list of drugs banned from competition, these "women" were able to pass all drug testing without any worries. Of course, I don’t suggest you raid your girlfriends birth control pill case in order to make yourself "feel like Deca". Recent studies at UCLA (9) have shown that different types of birth control pills have different androgenic capacity and can change the Olympic doping standard of testosterone to epitestosterone ratio of six to one with an increase of that ratio.
Are you confused yet? How can one of the most manly of anabolic steroids such as Deca Durabolin be considered a female hormone? How can female birth control pills be used as anabolic steroids? The simple answer to this question is that progesterone is best not considered as a female hormone, but as a hormone with properties somewhere in the middle between testosterone and estrogen. You can tweak the progesterone molecule slightly one way and have a hormone that is androgenic, or tweak it another way and be less androgenic or become more neutral in effect like the natural progesterone in the human body. Progesterone has its reputation as a female hormone due to its role in promoting pregnancy. But natural progesterone is still present in the male and also plays an important role in male physiology, but it has not yet been clearly elucidated. It should be noted that the "masculine" hormone nor-testosterone, that is the basis for the anabolic steroid Deca Durabolin, is actually found in highest concentrations in pregnant women (10).
So how can progesterone like molecules make me big or improve my athletic performance? Are large doses of Deca what you are referring to when you talk about "progesterone for men"? The answer is that nortestosterone drugs and prohormones have disadvantages over testosterone for use in hormone replacement therapy and in athletics / bodybuilding. The main reason nortestosterone is so popular is because of its lower androgenicity. It competes with testosterone for the 5-alpha reductase enzyme that converts testosterone to DHT and instead converts to dihydronortestosterone which is much less androgenic. Therefore you are less likely to experience side effects often associated with testosterone such as acne, hair loss, etc.
However, some people don’t know about nor understand the drawback of nortestosterone. For one thing, it can drastically lower libido. This is not surprising since other progestin based drugs are given to sex offenders to purposely lower their libidos. For male hormone replacement therapy, this can make nortestosterone a big no-no. Most men considering hormone replacement therapy are already suffering from a loss of libido, and nortestosterone can be almost like a castration agent for them. In addition, nortestosterone has a lower aromatization rate than testosterone. Since estrogen can raise HDL levels while androgens tend to lower HDL, this lack of estrogen from nortestosterone can cause HDL levels to drop further than when on testosterone. While temporarily low HDL levels may not be a big concern for a healthy young athlete, this is obviously a bigger concern for older men or those with heart disease risk factors.
Instead of using nortestosterone for hormone replacement therapy, I recommend a combination of natural testosterone and pulses of natural progesterone when testosterone is used. Progesterone, like nortestosterone, competes with testosterone for the 5-alpha reductase enzyme. A combination of testosterone/progesterone could allow for the benefits of increased testosterone while keeping DHT levels balanced. The concept is to help maintain a natural and youthful testosterone/estrogen/progesterone ratios throughout your lifetime. I believe a proper balance is the key to a healthy libido, prostate, and cardiovascular system.
While synthetic progesterone derivatives have been used to lower libido in men (1, 5), I believe that natural progesterone may in fact have the opposite effects in some men. I have heard patient anecdotes and from other medical doctors saying that application of a natural progesterone cream to the scrotum can increase libido and enhance orgasmic pleasure in some men.. I believe that just as high doses of synthetic progesterone derivatives can lower libido, so can low levels of natural progesterone. Natural progesterone can have a calming effect on the nervous system and may help those men who are "rapid ejaculators" or have other anxiety related sexual problems. Restoring or pulsing progesterone may enhance libido, and sexual function. While large doses of synthetic progestins may cause you to get fatter or lose muscle, the role of progesterone in increasing body temperature has been well studied in women (3) and may help bring back resting metabolic rate to a more youthful levels in men as well. Progesterone has the benefit of boosting metabolism but too much can lead to high insulin levels which would likely cancel out any benefits of increased metabolism.
Conclusion: The Future of "Contrarian Endocrinology"
I believe that the effects of "female" hormones on men have been greatly over demonized and understudied and there are many benefits to be derived both for body compositioning and for anti-aging purposes. Just as testosterone use effects women more noticeably than men, other hormones found in smaller amounts in men such as estrogen and progesterone can effect men more profoundly than women. Too much estrogen and progesterone will of course lead to loss of muscle mass, gains in fat, and loss of libido. But proper levels and more importantly the ratios of these hormones could actually be beneficial for libido and body composition.
While much more research needs to be done, I believe the best protocol for hormone replacement therapy for men will be quite similar to the one I use for women. As you may recall from Part I of this series, my usual protocol for female hormone replacement therapy is to restore a balanced ratio of testosterone/estrogen/progesterone through use of natural testosterone and progesterone gels and small doses of natural estrogens if necessary. I believe a good protocol for men may soon be a similar protocol of maintaining a natural balance of testosterone/estrogen/progesterone. Once a proper baseline level of sex hormones is achieved through use of natural gels, both men and women may desire an additional spike in energy or libido from time to time. For this purpose, occasional use of the short acting forms of the prohormones such as androstenedione or 4-androstenediol can be extremely effective in causing temporary boosts of testosterone and estrogen without disrupting your hormonal balance.
As for body compositioning and athletics, young adult men (not teenagers) can probably benefit most from spiking estrogen levels occasionally since they may have the lowest estrogen levels to begin with. Young adult men may also wish to increase muscle mass through nortestosterone or nortestosterone prohormones, since these should have less androgenic side effects. Older men should probably avoid nortestosterone and should instead use natural testosterone with a small amount of natural progesterone pulsing to minimize androgenic side effects.
I am eagerly looking forward to the day when my ideas are no longer considered "contrarian". As advanced as medicine is in the United States, the American medical establishment and the media often act like emotional and irrational children when it comes to sex hormone research. Testosterone and other androgens have been highly politicized and demonized in the past to the point where no constructive research could be done. Only recently has the importance of estrogen and natural progesterone in men and testosterone in women is starting to be looked at seriously. As more and more research is done, I am highly confident that it will be shown that keeping all of these three main sex hormones balanced throughout your lifetime can both extend life as well as improve the quality of your life.
References
-
Berlin FS. "Chemical castration" for sex offenders. N Engl J Med. 1997 Apr 3;336(14):1030
-
Crenshaw, Theresa L. ,With Goldberg, James P. "Sexual Pharmacology; Drugs That Affect Sexual Functioning", W. W. Norton & Company, March 1994
-
Cagnacci A, et al " Regulation of the 24-hour Rhythm of Body Temperature in Menstrual Cycles with Spontaneous and Gonadotropin-induced Ovulation" Fertil Steril. 1997 Sep;68(3):421-5.
-
Gladkova AI. "The Regulation of Male Sexual Behavior by the Sex Hormones"
Usp Fiziol Nauk. 1999 Jan-Mar;30(1):97-105
-
Kiersch TA. "Treatment of Sex Offenders with Depo-Provera." Bull Am Acad Psychiatry Law. 1990;18(2):179-87
-
King, DS, et al, "Effect of Oral Androstenedione on Serum Testosterone and Adaptations to Resistance Training in Young Men: a Randomized Controlled Trial". , JAMA. 1999 Jun 2;281(21):2020-8
-
Mauvais-Jarvis P, et al. "Inhibition of Testosterone Conversion to Dihydrotestosterone in Men Treated Percutaneously by Progesterone" J Clin Endocrinol Metab. 1974 Jan;38(1):142-7.
-
McEwen BS.," Clinical Review 108: The Molecular and Neuroanatomical Basis for Estrogen Effects in the Central Nervous System." J Clin Endocrinol Metab. 1999 Jun;84(6):1790-7
-
Personal communication to K. Ullis, M.D. from UCLA Sports Medicine researchers.
-
Reznik Y, et al. Rising plasma levels of 19-nortestosterone throughout pregnancy: determination by radioimmunoassay and validation by gas chromatography-mass spectrometry.
-
Thorneycroft IH. "Update on Androgenicity" Am J Obstet Gynecol. 1999 Feb;180(2 Pt 2):288-94 "
-
-
@wester130 The user you quoted has no understanding of chemistry.
19-Nortestosterone / Nandrolone, has very little structural similarity to progesterone - it is structurally similar to testosterone - it is simply testosterone, that is missing a methyl group on the 18th carbon of the molecule. Both nandrolone and progesterone both have keto groups on position 3 and a double bond on carbon 4, but that's the only similarity they have.
Nandrolone is an "estrane" whereas progesterone is a "pregnane".
Progesterone:
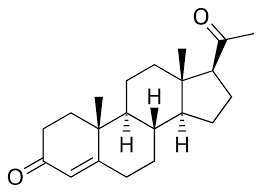
Nandrolone
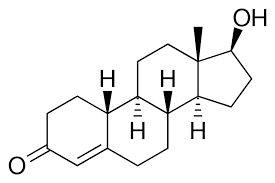
Testosterone:
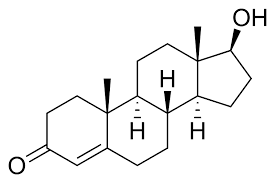
Nandrolone / 19-nor testosterone and it's derivatives are extremely potent agonists of the androgen receptor and the progesterone receptor.
But the latter doesn't make 19-nor testosterone derivatives anabolic; nandrolone has an affinity to the AR 50% more than testosterone. This is why it is more anabolic.
Progesterone itself actually acts as a mild glucocorticoid; binding to & activating it's receptor. It increases the transfer of amino acids out of muscle tissue - just search up "progesteorne binding affinity to the glucocorticoid receptor" and "progesterone tryrosine amino transferase" - plenty of in vivo and in vitro studies show that that progesterone itself is a mild glucocorticoid.
In some rodents, progesterone is a precursor to testosterone and can be anabolic via that pathway, but in humans at least, progesterone doesn't increase testosterone and is not anabolic.
-
Essentially, yes. But, they don’t disclose how much 4-androstenediol is in each capsule. Instead they call it something like “cyclo 4-diol”I would have to email them to find out if 4 androstenediol is even in it.
-
After a couple days of research, I also found a lot of positive reviews for a newly developed hormone that does not require enzymatic conversion and that is according to many older users on AnabolicMinds forums and r/prohormones reminiscent of old school prohormones.
It’s called “3-AD”. It is 5-androstenediol with the hydroxyl on 3 at alpha position instead of beta. They call it “dehydroandrosterol”, but chemically it is what I described.
What the logs specifically mention is the muscle hardness and pumps being like oral steroids like dbol.
-
This post is deleted!