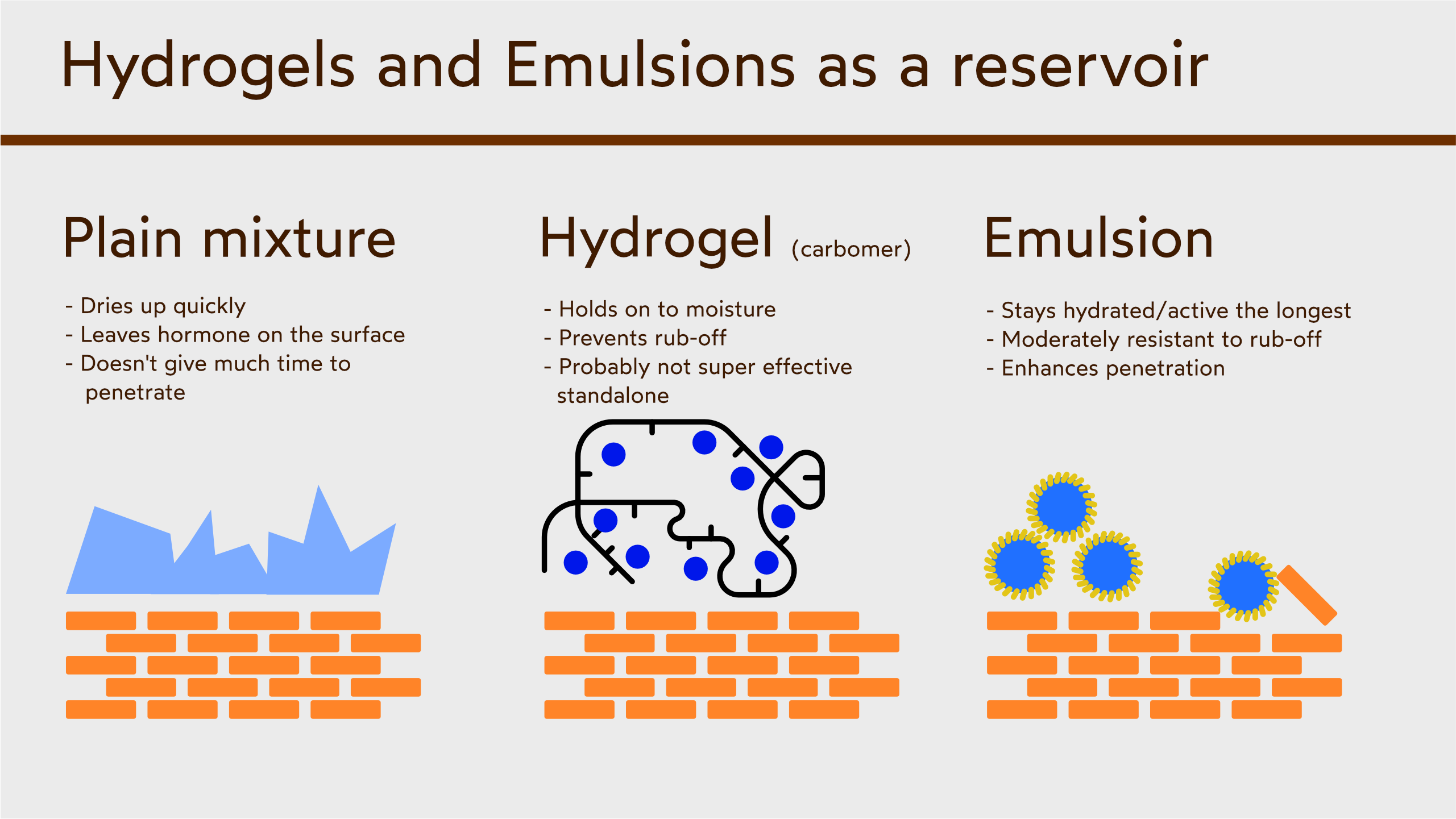
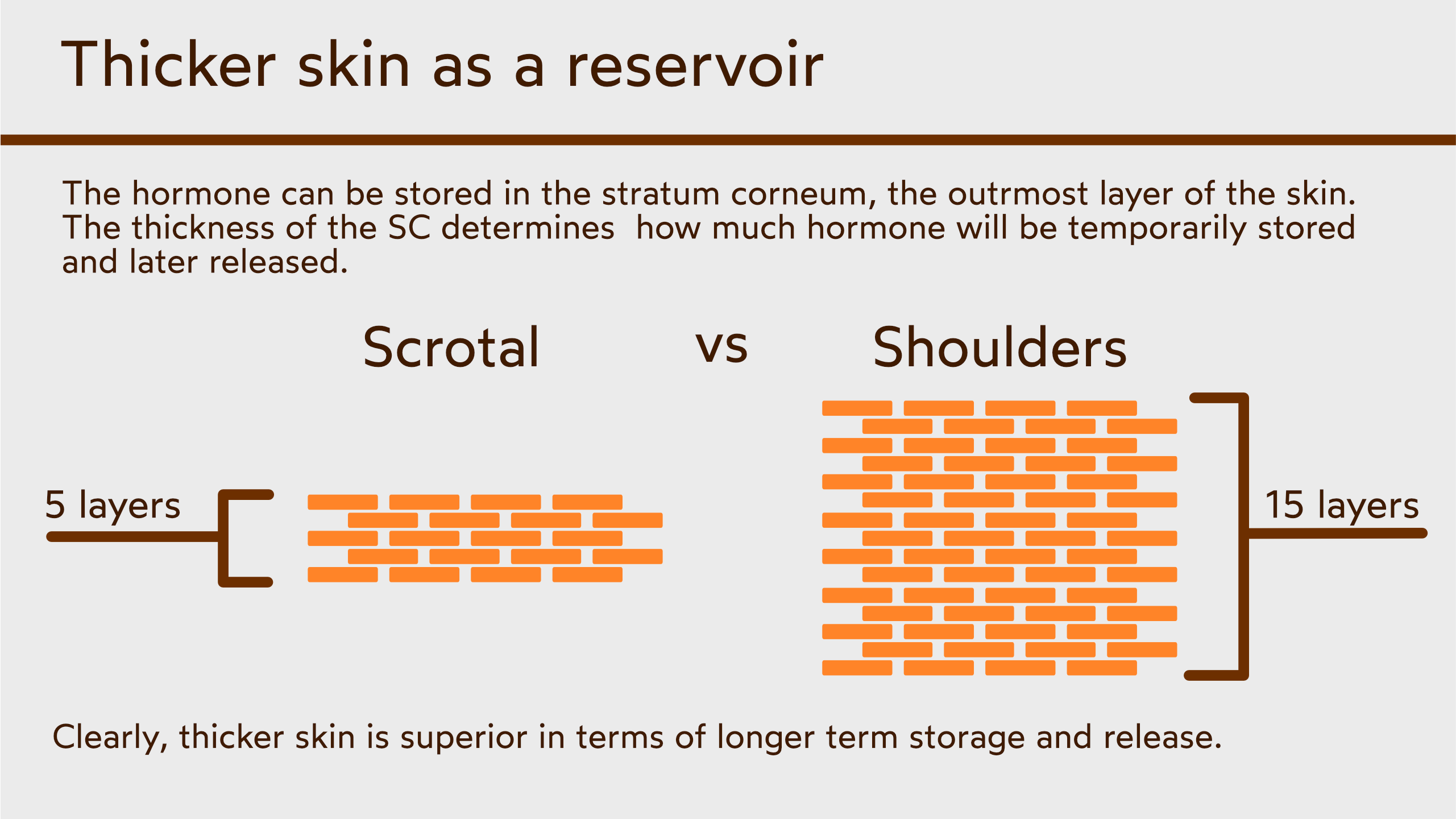
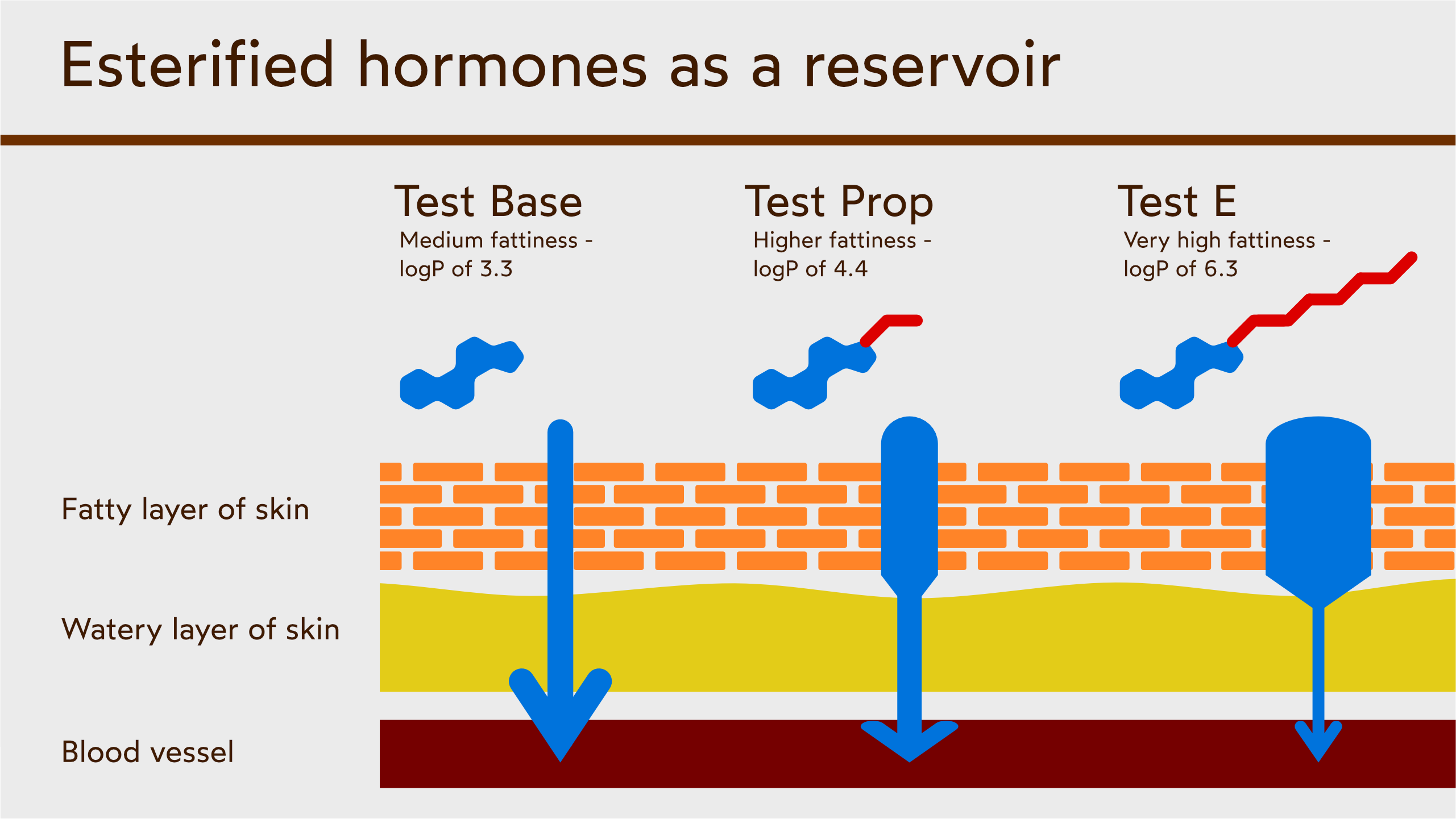
Experiments with transdermal hormones
-
@metabolicmilk Since you're using less common solvents and a long list of excipients, you'll need to make sure every excipient is fully miscible with each other and that your active ingredient dissolves properly in the solvent.
DMI and Propylene Glycol aren’t widely used in labs, so there’s little to no published data on how well hormones like testosterone dissolve in them. And when it comes to other excipients like squalane and IPM, there’s absolutely no data at all on how they interact with DMI or Propylene Glycol. Additionally, there is no data on how IPM interacts with squalane, for example.
Chat GPT is very, very bad at estimating data. It won't estimate figures unless you force it to, for this precise reason. Therefore, ignore its solubility and penetration figures.
The only realistic way you may achieve this is ordering an excess of all of these ingredients and figuring out the miscibility of the active ingredient and excipients by trial and error; and even if you do this, you will have no idea if the active ingredient exists as crystals inside the gel, or if it is dissolved inside the solvent. Pharmaceutical gels are designed to have the active ingredient to be either crystalline or dissolved in the gel; when it's crystalline, the crystals will sit on top of the skin and absorb into the skin, creating a more local effect. When the API is dissolved in the gel, it goes systematic.
Even if you do this, it might simply not work. My recommendation is this: injectable testosterone propionate will offer testosterone that has a 19-hour half-life, quicker to apply than a gel, cheaper, MUCH more convenient (you don't have to make it), and it is researched, therefore, you will know exactly how much testosterone will be going into your blood stream and for how long. It won't hurt to inject if you use a 27g needle, in your buttocks, therefore, I am willing to say it may be more comfortable to use.
-
@alfredoolivas said in Experiments with transdermal hormones:
Since you're using less common solvents and a long list of excipients, you'll need to make sure every excipient is fully miscible with each other and that your active ingredient dissolves properly in the solvent.
DMI and Propylene Glycol aren’t widely used in labs, so there’s little to no published data on how well hormones like testosterone dissolve in them. And when it comes to other excipients like squalane and IPM, there’s absolutely no data at all on how they interact with DMI or Propylene Glycol. Additionally, there is no data on how IPM interacts with squalane, for example.
Chat GPT is very, very bad at estimating data. It won't estimate figures unless you force it to, for this precise reason. Therefore, ignore its solubility and penetration figures.
The only realistic way you may achieve this is ordering an excess of all of these ingredients and figuring out the miscibility of the active ingredient and excipients by trial and error; and even if you do this, you will have no idea if the active ingredient exists as crystals inside the gel, or if it is dissolved inside the solvent. Pharmaceutical gels are designed to have the active ingredient to be either crystalline or dissolved in the gel; when it's crystalline, the crystals will sit on top of the skin and absorb into the skin, creating a more local effect. When the API is dissolved in the gel, it goes systematic.
You’ve raised some good points :
Miscibility Concerns:
I plan to conduct small-scale miscibility tests for all solvent/excipient combinations before scaling up. If phase separation occurs, I’ll explore emulsifiers or adjust ratios to achieve homogeneity.Solubility:
propylene glycol (PG) is well-documented for steroids like testosterone, with solubility typically ranging from 50–100 mg/mL. DMSO is also widely used and can dissolve testosterone at high concentrations (>200 mg/mL).Crystalline vs. Dissolved API:
If undissolved crystals are present, I’ll adjust the solvent blend or increase co-solvent ratios. -
Yo! Red Button! My Man! How you been ?
-
@JamesGatz yo nigga, check pms
-
I just did a batch
50% ethyl alcohol
30% IPM
10% T Base
10% DMSOPowder dissolved very well, super clear.
But I forgot to thinking about the interaction between DMSO and ethyl alcohol
Will I get alcohol poisoning lol?
Should I dump this
-
@yeyo12 No lol, it's safe.
-
Thank you, not sure if I notice any different anyway, been doing transdermal T with different formulas in a while.
Now doing forearms and back of knees. I was doing shoulders but I think alcohol irritated the skin there so I changed to forearms.
I just got DMSO so this is the first batch with DMSO. We'll see how it goes
-
@brightside Sorry for the bump but how did you purify exemestane from pills?
I got my hands on some and am eager to try transdermal to not waste it taking orally. -
@PissBoy the bioavailabilty of exemestane is around 50% if I rememember correctly, and if you take it with a fatty meal, it increases by around 30%, giving you around 80% absorption.
It is a modified version of 1,4-androstenedione that is designed to have high oral bioavailability, long half life and no hepatoxcity. It converts into a drug very similar to boldenone inside the body.
You will likely loose more exemestane trying to extract it in a solvent. Furthermore, it likely contains silicon dioxide and titanium dioxide which might be soluble in the solvents.
-
@alfredoolivas Also, given how little the effective dosage is (2.5-7.5 mg), there is virtually no point in administering it in a way that gives higher bioavailability.
-
@alfredoolivas the Pfizer exesmastane 25mg pills or whatever they are are insanely hard to slice up ime
-
@alfredoolivas According to the wiki page on exemestane, exemestane succeeds as an aromatase inhibitor because its metabolites competitively bind to aromatase (in place of aromatase's 'natural' substrate 4-androstenedione), rendering the aromatase enzyme inactive.
That description makes it seem as though testosterone cannot aromatize without converting to androstenedione first. If that is true, it is notable.
Second, the oral bioavailability of exemestane is high - the wiki page claims 60%. I'm not sure if that means 60% of oral exemestane survives first pass metabolism OR that 60% of the oral exemestane is converted to active metabolites after first pass metabolism. I strongly believe the latter is the correct interpretation.
Finally, the structure of exemestane and its lack of hepatotoxicity makes it blaringly obvious how easy it is to develop a non-17a alkylated orally active AAS. You just need a tiny bit of thinking to figure it out.
-
@eduardo-crispino Use a pill cutter, I have cut them succesfully using one
-
@jamezb46
There is some confusion here I think, due to the wikipedia page@jamezb46 said in Experiments with transdermal hormones:
According to the wiki page on exemestane, exemestane succeeds as an aromatase inhibitor because its metabolites competitively bind to aromatase (in place of aromatase's 'natural' substrate 4-androstenedione), rendering the aromatase enzyme inactive.
Okay so the 17-beta hydroxy metabolite of 6-methylene-androsta-1,4-dien-3-one binds to the aromatase enzymes and inactivates it?
@jamezb46 said in Experiments with transdermal hormones:
That description makes it seem as though testosterone cannot aromatize without converting to androstenedione first. If that is true, it is notable.
But this suggests that testosterone, the 17-beta-hydroxy metabolite of androstenedione cannot bind to the aromatase enzyme?
These statements are contradictory to my understanding, please feel free to explain them further.
Exemestane doesn't have to metabolise to inhibit aromatase:
Exemestane showed a higher aromatase affinity than 10 (Ki=26 and 92 nM, respectively) and a faster enzyme inactivation@jamezb46 said in Experiments with transdermal hormones:
Second, the oral bioavailability of exemestane is high - the wiki page claims 60%. I'm not sure if that means 60% of oral exemestane survives first pass metabolism OR that 60% of the oral exemestane is converted to active metabolites after first pass metabolism. I strongly believe the latter is the correct interpretation.
Exemestane's bioavailability is 60%.
"Exemestane is extensively metabolized, with levels of the unchanged drug in plasma accounting for less than 10% of the total radioactivity after administration of radiolabeled exemestane to healthy postmenopausal women (CExemestane is extensively metabolized, with levels of the unchanged drug in plasma accounting for less than 10% of the total radioactivity after administration of radiolabeled exemestane to healthy postmenopausal women (Clemett and Lamb, 2000). P)"
-
@jamezb46 said in Experiments with transdermal hormones:
Finally, the structure of exemestane and its lack of hepatotoxicity makes it blaringly obvious how easy it is to develop a non-17a alkylated orally active AAS. You just need a tiny bit of thinking to figure it out.
Given it converts into 6-methylene-boldenone which has a strong affinity to the androgen receptor, with a 24 hour half life I believe, I would love to see if it has anabolic effects. It has been shown to have anabolic effects in bones.
Currently, I am taking 50mg of exemestane powder a day, so I should be getting some 6-methylene-boldenone
The addition of the methyl group onto position 17 of androgens makes them much more anabolic and activators of the AR, if I rememember correctly, so perhaps the 17-alkylation of steroids has more effects than increasing it's half life and bioavailability
-
@alfredoolivas No joint pain at 50mg?
You're probably taking DHEA or prog/preg too?
Also Im still not sure about the oral bioavailability:"EXE is characterized by high lipophilicity, poor solubility in water, limited dissolution in the gastrointestinal milieu, extensive first-pass metabolism, and limited oral bioavailability (<10%) that restricts its clinical application against BC (Chaturvedi and Garg, 2022). " - link
Since it slowly builds up over a week of consistent daily dosing of 25mg does it make sense that taking less over a longer time would lead to a similar steady plateau at a similar efficacy?
Similar to how Creatine is dosed highly and slowly tapered off into a maintenance dose as you reach "saturation", -
@PissBoy Interesting, thanks for sharing. @jamezb46 the wikipedia page doesn't give a citation towards the 60% bioavailability!
I found this study too.
Preclinical data in animals (rats and dogs) when exemestane was administered via IV route (formulated in polypropylene glycol and saline 50:50 v/v) indicated that the absolute bioavailability was about 5%.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2802159/#:~:text=Preclinical data in animals (rats,absolute%20bioavailability%20was%20about%205%25.So maybe the extra carbon, attatched to the carbon on position 3 via a double bond, doesn't increase bioavailability. It just increases half life, perhaps it stops the 5 alpha reduction ?
Lol, I was taking 75mg of exemestane dissolved in DMSO last year. Assuming 100% absorption, I was taking the equivelent to 750mg of exemestane orally.
@PissBoy even if the bioavailability, is 10%, it doesn't matter. This further goes to show how powerful exemestane is. All the studies were done with oral exemestane, and even with this low bioavailability of less than 10%, and still, 2.5mg to 7.5mg of exemestane was able to lower estrone sulfate by around 70%.
https://pubmed.ncbi.nlm.nih.gov/7547212/
"...Exemestane is a novel orally active irreversible aromatase inhibitor and our study shows its effectiveness in suppressing both serum E1 (estrone) and E2 (estradiol) levels when given in repeated doses. A significant reduction in serum oestrone sulfate (E1S) oestrogen levels was also obtained with the lowest dose of 2.5 mg. The difference in the percentage of E2 reduction obtained with the lowest dose does not seem to be due to any lesser efficacy in inhibiting E2 synthesis, but more probably to the lower E2 baseline values of these patients. Our data also show an effective reduction of E1S levels in the 2.5 mg group, similar to that observed by Evans et al. (1992) using higher single doses."@PissBoy said in Experiments with transdermal hormones:
Since it slowly builds up over a week of consistent daily dosing of 25mg does it make sense that taking less over a longer time would lead to a similar steady plateau at a similar efficacy?
Similar to how Creatine is dosed highly and slowly tapered off into a maintenance dose as you reach "saturation",Yes.
Multiple studies have shown more is not better. On a side note it is beautiful to read about a steroid that is actually heavily researched and clinically researched, lol, no conjecture has to be made @jamezb46
https://pubmed.ncbi.nlm.nih.gov/9815789/
Exemestane (10 mg o.d.) caused maximal suppression of plasma estradiol (E2) and estrone (E1) to a mean of 14.6 and 5.8% of pretreatment levels, respectively, whereas 25 mg of exemestane o.d. suppressed estrone sulfate (E1S) to 8.9% of pretreatment levels. No fall in adrenal steroid levels was recorded. Exemestane (5 mg o.d.) suppressed urinary E2 and E1 to a mean of 11.9 and 12.2% of pretreatment levels, respectively. Administering exemestane at doses of 50-200 mg o.d. caused no further suppression of urinary E1, whereas urinary E2 fell to 6-7% of pretreatment levels. "https://jamanetwork.com/journals/jamaoncology/fullarticle/2802824
Conclusions and Relevance In this randomized clinical trial, exemestane, 25 mg, given 3 times weekly in compliant patients was noninferior to the once-daily dosage in decreasing serum estradiol. This new schedule should be further studied in prevention studies and in women who do not tolerate the daily dose in the adjuvant setting.
-
Lol, I was taking 75mg of exemestane dissolved in DMSO last year. Assuming 100% absorption, I was taking the equivelent to 750mg of exemestane orally.
And if 2.5mg oral is enough to surpress estrogen significantly, by this logic 0.25mg of EXE in DMSO would do the same. Sounds dreamy, but I cant really find any study about exemestane transdermal application that I can actually decipher lol.
Thank you for your answers.
-
are you going to buy and experiment with Stanolabs DHT gel?
https://bioenergetic.forum/topic/3338/stanolabs-dht-gel?_=1747131281451
-
@wester130 no, I don’t trust steroid products that aren’t lab tested unless I trust the source. Especially rare products such as DHT which currently can’t be sourced anywhere, so it’s not like he can easily get DHT and sell it. I’d recommend him to get a DHT lab test from Janoshik, to help people with concerns like me. It would cost 120 dollars + EU shipping