Antibiotics causing fungal infections - mechanism is through reducing IL-17a and GM-CSF immune cells
-
https://www.sciencedirect.com/science/article/pii/S1931312822002190
IL-17A and GM-CSF {in cd4 T cells or Innate lymphoid cells} protect against antibiotic-induced susceptibility to invasive candidiasis
. antibiotics disrupt cytokine production from GI-tract-resident CD4 T cells and innate lymphoid cells (ILCs), particularly IL-17A, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF)
. IL-17A is a critical antifungal molecule at the mucosal barrier, as inborn errors of IL-17 immunity cause mucosal candidiasisWe found that antibiotic pre-exposed Il22−/− animals had greater mortality post-fungal infection compared with Il22−/− animals that did not receive antibiotics (Figure 3C), indicating that IL-22 is not required to mediate the susceptibility phenotype in this model.
By contrast, mice lacking either IL-17A or GM-CSF exhibited similar susceptibility to fungal infection regardless of antibiotic pre-exposureIn addition to IL-17A, we found that GM-CSF production was reduced by antibiotics and that the presence of GM-CSF was required for the development of antibiotic-induced susceptibility during invasive candidiasis. GM-CSF has been shown to have a protective benefit against invasive candidiasis in humans (Dignani et al., 2005; Gavino et al., 2014). We show here that GM-CSF can ameliorate mortality in the setting of antibiotic-associated invasive candidiasis in mice. GM-CSF treatment may be effective at boosting the antifungal activity of myeloid cells
IL-17A production by CD4 T cells is especially sensitive to antibiotic treatment, as we confirmed here. IL-17A is a key antifungal cytokine at mucosal sites since patients with IL-17 receptor signaling defects are susceptible to mucosal candidiasis and enhanced C. albicans GI colonization
Indeed, we found that IL-17A was required to control the C. albicans infection of GI tissues in the setting of antibiotic pre-exposure{they dont lower il-17a t cells directly - they lower the bacteria that helps induce IL-17 t cells "Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria"}
Another driver of gut Th17 cells is microbial-derived ATP. Both SFB and bacterial ATP are reduced by antibiotics, which has been linked to reduced IL-17A production.
vancomycin specifically enhanced the susceptibility to C. albicans following long-term oral treatment and only indirectly via dysbiosis -
@cs3000 said in Antibiotics causing fungal infections - mechanism is through reducing IL-17a and GM-CSF immune cells:
https://www.sciencedirect.com/science/article/pii/S1931312822002190
IL-17A and GM-CSF {in cd4 T cells or Innate lymphoid cells} protect against antibiotic-induced susceptibility to invasive candidiasis
. antibiotics disrupt cytokine production from GI-tract-resident CD4 T cells and innate lymphoid cells (ILCs), particularly IL-17A, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF)
. IL-17A is a critical antifungal molecule at the mucosal barrier, as inborn errors of IL-17 immunity cause mucosal candidiasisWe found that antibiotic pre-exposed Il22−/− animals had greater mortality post-fungal infection compared with Il22−/− animals that did not receive antibiotics (Figure 3C), indicating that IL-22 is not required to mediate the susceptibility phenotype in this model.
By contrast, mice lacking either IL-17A or GM-CSF exhibited similar susceptibility to fungal infection regardless of antibiotic pre-exposureIn addition to IL-17A, we found that GM-CSF production was reduced by antibiotics and that the presence of GM-CSF was required for the development of antibiotic-induced susceptibility during invasive candidiasis. GM-CSF has been shown to have a protective benefit against invasive candidiasis in humans (Dignani et al., 2005; Gavino et al., 2014). We show here that GM-CSF can ameliorate mortality in the setting of antibiotic-associated invasive candidiasis in mice. GM-CSF treatment may be effective at boosting the antifungal activity of myeloid cells
IL-17A production by CD4 T cells is especially sensitive to antibiotic treatment, as we confirmed here. IL-17A is a key antifungal cytokine at mucosal sites since patients with IL-17 receptor signaling defects are susceptible to mucosal candidiasis and enhanced C. albicans GI colonization
Indeed, we found that IL-17A was required to control the C. albicans infection of GI tissues in the setting of antibiotic pre-exposure{they dont lower il-17a t cells directly - they lower the bacteria that helps induce IL-17 t cells "Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria"}
Another driver of gut Th17 cells is microbial-derived ATP. Both SFB and bacterial ATP are reduced by antibiotics, which has been linked to reduced IL-17A production.
vancomycin specifically enhanced the susceptibility to C. albicans following long-term oral treatment and only indirectly via dysbiosisHow do you upregulate those after a course of antibiotics ?
-
@Santosh How do you upregulate those after a course of antibiotics ?
not sure wondering too, would be useful to know
maybe if people target ways to bring back certain strains is 1 way, but obviously wouldnt be useful during taking them & might take a while
Here we report that specific members of the commensal microbiota known as segmented filamentous bacteria (SFB), with the candidate name Arthromitus, are potent inducers of Th17 cells in the SI LP of mice. SFB, spore-forming gram-positive bacteria most closely related to the genus Clostridium, have been reported to colonize the intestines of numerous species, including humans
Introduction of SFB, but not other bacteria, into Th17 cell-deficient mouse models induced IL-17 and IL-22 expression in CD4+ T cells in the SI LP -
Isnt IL17 pro inflammatory ?
L reuteris mechanism is that it lowers IL17 and increases Il10. -
I personally was thinking phages.
-
@Mauritio said in Antibiotics causing fungal infections - mechanism is through reducing IL-17a and GM-CSF immune cells:
Isnt IL17 pro inflammatory ?
L reuteris mechanism is that it lowers IL17 and increases Il10.That's one hell of a study . Their testosterone skyrocketed and the size of their balls is kind of comical ... And it all depends on antagonizing IL17.
"We tested whether these features typical of sustained reproductive fitness may be due to anti-inflammatory properties of L. reuteri, and found that testicular mass and other indicators typical of old age were similarly restored to youthful levels using systemic administration of antibodies blocking pro-inflammatory cytokine interleukin-17A. This indicated that uncontrolled host inflammatory responses contributed to the testicular atrophy phenotype in aged mice. "
BTW L. Reuteri secretes a molecule called reuterin, which is antifungal as well.
-
I think taking rivoflavin can help avoid the opportunistic fungal infection after antibiotics.
Also, maybe it's a good idea to take some flowers of sulfur after the round of antibiotics is done?
-
@Mauritio it gave them baseball nuts lol . reminds me of that study where they gave rodents a bacteria from an olympian and it gave them superpowers
yeh proinflammatory as usual way for immune system to eliminate fungus according to op study.
i tried reuteri a while back, think it was the strain that starts with 6 , took it with glucose. i wrote in notes "good to feed with glucose but lactose boosts reuterin production more". but i didnt notice any changes for energy mood or inflammation, but wasnt trying for low test specifically@tea nice the riboflavin checks out , great study https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9927497/ 1mg/kg i.p , shows it reduced a lot on the tongue and more in kidneys. i think u can absorb up to ~30mg orally
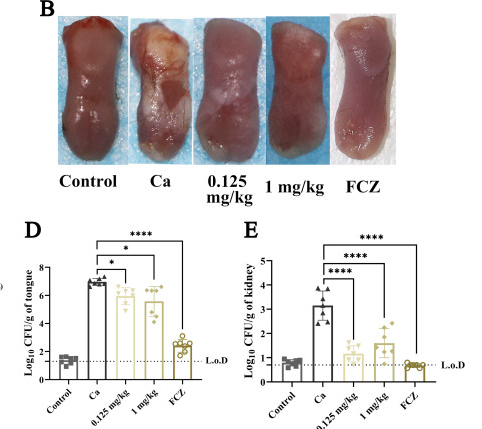
Interestingly it stopped the fungus transporting & processing carbs/sugar , pyruvate accumulated