A combination of vitamin B1/B3/B7 and aspirin, has curative effects on human mantle-cell lymphoma
-
@Ismail hey bud, yep, been working through that interview in pieces all day. One of the best 2 hour podcasts I’ve ever listened to. Just wall to wall information.
-
@evan-hinkle haha same my man
 an extremely beneficial episode - trying to finish it off, life keeps getting in the way
an extremely beneficial episode - trying to finish it off, life keeps getting in the way 


-
@haidut
amazing .something extra (lipoic acid as another cofactor for PDH)
https://www.tandfonline.com/doi/full/10.4161/cbt.22003
https://www.frontiersin.org/articles/10.3389/fnagi.2020.00262/full -
@DavidPS said in A combination of vitamin B1/B3/B7 and aspirin, has curative effects on human mantle-cell lymphoma:
@Mauritio - Yes, it appears that he is not interested in patenting.
He selected the least expensive form of B1 to make his invention accessible to as many people as possible.
I am a registered patent attorney. I think he could obtain a patent based on the limited facts that I have. He solved a long-standing problem; that is a strong indication of patentability.
Ok in that case ,you know more about this than me.
Would be an interesting thing to ask him if he has any intentions on patenting it. -
@Ismail said in A combination of vitamin B1/B3/B7 and aspirin, has curative effects on human mantle-cell lymphoma:
Anyone watched Georgi’s interview on the strong sistas channel? He goes into discussing his study in quite some depth:
Thanks for posting , I was going to ask where he talked about it.
I'll watch it soon, but does he mention how he came on with using dihydrobenzoic acid ?
IIRC his methylene blue product contains benzoic acid ... -
I'm surprised Georgi didn't include B2 into the mix as well due to it's FNM/FAD qualities. The results are impressive nonetheless, but unless he's got a good reason for not including it I don't see why a human trying to replicate this couldn't do a B1/B2/B3/B7 + aspirin combo.
-
@Mauritio talks about it at 21 mins (worked better than aspirin so far)
aspirin + 2,6 dihydroxybenzoic acid stopped progression but didnt regress,
2,6 alone without aspirin or b vits stopped & already showed regression
vitamins + aspirin or vitamins + 2,6 are in between, with the vitamins + 2,6 working better[btw in vivo benzoic acid as sodium benzoate has toxicity when you go to high milligram doses,
it crashes cholesterol , crashes blood cells induces anemia (weirdly after an initial increase) (maybe through lowering ceruloplasmin), skews electrolytes after 1 week, at dose ~200mg and ~600mg
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2862570/bin/nihms193118f7.jpg
https://www.researchgate.net/publication/268298860_Effect_of_oral_intake_of_sodium_benzoate_on_some_haematological_parameters_of_wistar_albino_ratsmaybe the 2,6 form works different , (id think so because if it was crashing red blood cells significantly that would play into less co2 production and hinder the positive outcome)
-
Thanks.
So the way I heard it, the following order emerges in terms of effectiveness, from high to low (mind you, the experiments are still ongoing):- 2,6 dihydroxybenzoic acid
- 2,6 dihydroxybenzoic acid + B Vitamins
- Aspirin + B Vitamins
- Aspirin + 2,6 dihydroxybenzoic acid
-
What is not clear to me is why the 2,6 dihydroxybenzoic acid is more effective without the vitamins, since they should only enhance the anti-cancer effect...
-
This is 2,6 dihydroxybenzoic acid :
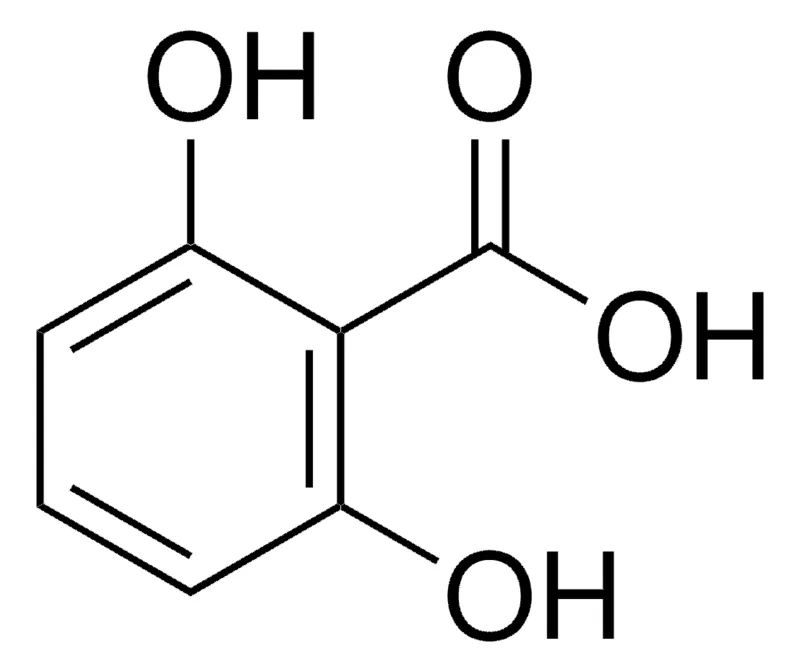 <img src="https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/structures/196/724/160fee6b-8d74-427c-b54d-738933a5fa1c/800/160fee6b-8d74-427c-b54d-738933a5fa1c.png" alt="2,6-Dihydroxybenzoesäure 98%"/>
<img src="https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/structures/196/724/160fee6b-8d74-427c-b54d-738933a5fa1c/800/160fee6b-8d74-427c-b54d-738933a5fa1c.png" alt="2,6-Dihydroxybenzoesäure 98%"/>this is aspirin (acetyl salicylic acid):
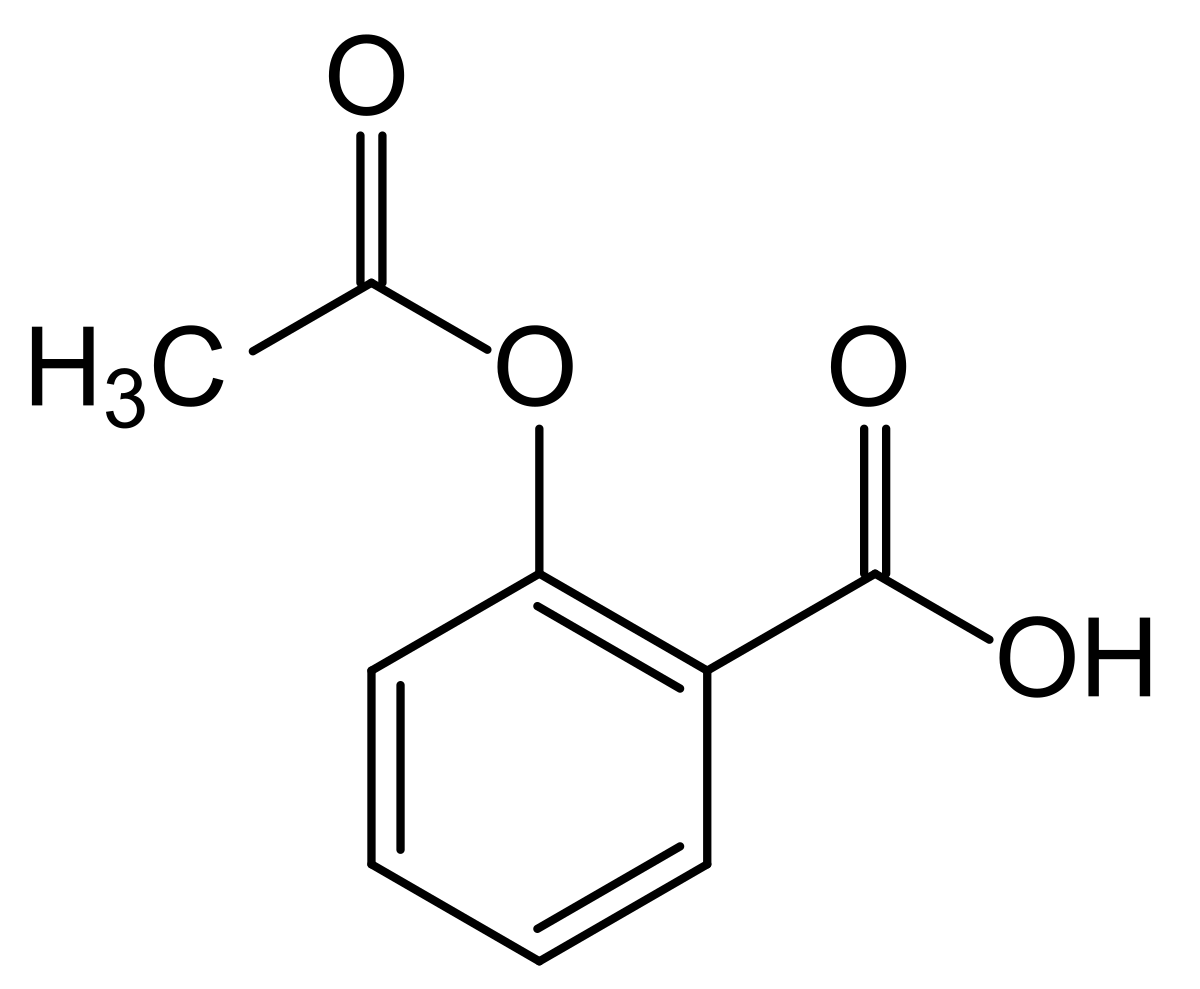
The structural similarity becomes even more obvious when you look at salicylic acid, which is where aspirins effects come and is called 2-hydroxybenzoic acid:
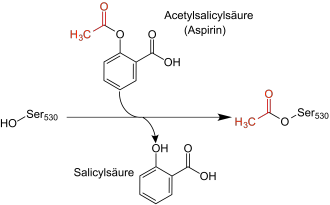 <img<img
<img<img -
@Mauritio yeh thats odd
curious how well the b vits do alone (how much of the aspirin / 2,6 effect relies on extra carbonic anhydrase inhibition or a different mechanism if thiamine covers that?0extra carbonic anhydrase II inhibitors at nanogram concentration (as long as they dont mess with other aspects of mitochondria)
https://www.tandfonline.com/doi/full/10.3109/14756366.2011.593176 -
@Mulloch94 I thought the same; I can only surmise he was trying to use the least amount of ingredients to achieve the desired result/outcome

 ️
️@haidut fair assumption?
-
@Ismail Probably so, I think if any of them are expendable it's probably B2, or B7. Both thiamine and niacin seem to be the "heavy-hitters" of the B vitamins.
-
@cremes said in A combination of vitamin B1/B3/B7 and aspirin, has curative effects on human mantle-cell lymphoma:
@evan-hinkle said in A combination of vitamin B1/B3/B7 and aspirin, has curative effects on human mantle-cell lymphoma:
BTW, I'm serious about the latter part. As bioenergeticists, we believe that most/all diseases are brought about by broken or sub-optimal metabolism. If it's true, then this protocol should also serve to potentially solve type2 diabetes, heart disease, hypertension, IBS, dementia, alzheimers, OBESITY, and autoimmune diseases like MLS, Lupus, etc.
I have heart disease. I'm going to try this protocol and see what happens. I'm already taking B1, low-dose B3, and aspirin daily. I just need to adjust the amounts a bit and add in some biotin.
This morning I have started this protocol. If this helps restore my oxidative phosphorylation to youthful levels then not only does this cure cancer but it should broadly cure other metabolic diseases.
30 days starts now. I will re-evaluate ~June 15 assuming I don't run into an issue that forces me to abandon this early.
-
@cremes good luck! Look forward to hearing your updates

-
Yes @cremes and anyone else, good luck!
It does seem like a potential reset for some. Dosages may vary for certain people with different conditions imo. And a reminder to not deplete the other B vitamins or certain minerals while performing this protocol.
-
Great Postings, @haidut and all here. Just a quick finding when I was checking (before travelling and buying food/supplies) on the internet some frozen fish at semi-nearby shops..... Seems the preservative 223 = Sodium Metabisulfite is used on frozen prawns/fish/seafood. !!! I was going to buy some frozen prawns (from Northern Australia), but has this additive.
 So this form of Sulfite (or it's cousins in the same range) are just another additive to the already abundant use in the food industry.
So this form of Sulfite (or it's cousins in the same range) are just another additive to the already abundant use in the food industry.This below relates to the thread and the lack of B vitamins or the lack of the potential function of B vitamins in one's diet!
from https://www.fedup.com.au/factsheets/additive-and-natural-chemical-factsheets/220-228-sulphite-preservativesSulphites destroy thiamine (Vitamin B1) so some experts recommend that foods which are a significant source of thiamine, such as meats, dairy foods and cereals should not be sulphited. In Australia, a number of pet cats and dogs have died from thiamine deficiency due to a steady diet of pet meat containing unlisted sulphites. Since sulphites cleave the thiamine molecule, thiamine in vitamin supplements can also be destroyed by sulphites. For this reason, in the USA there has been a total prohibition on the use of sulphites in meats since 1959, although sulphited meats such as sausages are still widely eaten in other English and Spanish speaking countries. Sulphites are also thought to destroy folic acid.
-
It's been a tad over 2 weeks since I started the protocol. I weight about 100kg so the math is really easy. I take 3 grams niacinamide, 1.5g thiamin Hcl, 1.5g aspirin, and 300mg biotin/b7 each morning. The aspirin is crushed into my morning Earl Grey tea along with 2 tbsp of honey. I eat the pills with water while the tea is steeping and then drink the tea over the course of about 20 minutes.
A few observations...
-
I feel better
As a heart attack survivor of ~2 years, the first 18 months of it I had to get used to being out of breath from climbing stairs to the second story of my house. This annoyed me but I was acclimating. Since starting the protocol, my breathlessness has eased significantly. -
Warmth
I already take ~20mg of kuinone/K2 and 3x 2 drops of Tyromix each day. My temperatures were improving but were still sub-98F. Since starting the protocol, my waking temp is 98.4F routinely and during the day as I eat my ~300g of carbs I am measuring 99.4F at times.
I have not noticed any weight loss or body recomposition.
Will continue for at least 2 more weeks and then I'll decide if I will extend for another 30 days.
-
-
@cremes It has now been another 30 days. I've remained on the protocol and feel mostly fine. Temperatures are still good. Weight is unchanged. Body comp is unchanged.
I've been seeking and getting ~7k IU of midday sun per day so I'm getting a nice tan. I have not noticed better sleep, weight loss, or other benefits. I assume my vit D status is low and I am now rebuilding it. I'll likely take a vitD blood test soon so I know where I'm at.
For a while there I thought maybe this protocol was improving some skin tags I have near my left and right armpits. However, while one or two tags "died off" the others are unchanged. I also had a mole on my right quad which has shrunken to almost nothing in the past 2 months. I am unsure if this is due to the protocol or due to the sun exposure.
I'm going to go another 30 days for a full 90 and then re-evaluate. I'm almost certain to take a break at that point. I imagine I should wean myself down over the course of 3-5 days instead of quitting everything cold turkey.
Sorry I don't have stronger benefits to report. I am unhappy about that too.
-
@cremes thank you for the updates, genuinely much appreciated
