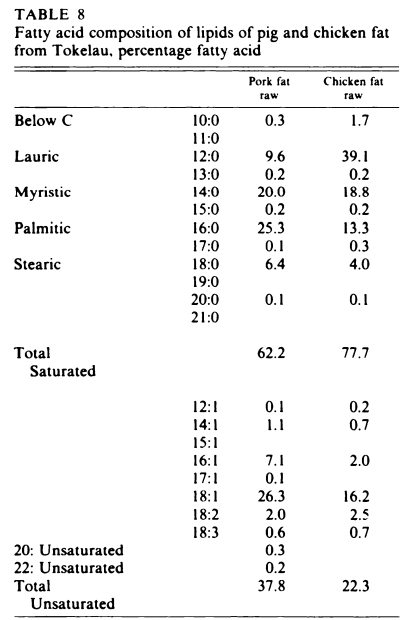
tl;dr - Iodinating (or halogenating) the double bounds of unsaturated fatty acids makes them behave more like saturated fats than unsaturated fats.
My goal is a slightly prolific introduction for those who are interested, since I feel that this side ends up losing a bit of potential by limiting iodine only to the thyroid (or more timidly to iodocasein). The great affinity of molecular iodine (I2) for the double bounds of PUFAs should cause some curiosity, since the affinity is such that unsaturation is usually determined by the iodine value. The idea that the complexity of today's organisms originated in the ocean, where the more unsaturated PUFAs such as EPA, DHA and ARA predominate, in my opinion only reinforces that this affinity is not random.
My interest in this subject began when I read Travis from the old forum mentioning that iodinated PUFAs behaved more like saturated fatty acids; an iodinated PUFA couldn't even be used to produce eicosanoids.
Since the double bond is obliterated by added iodine atoms, the lipids' geometry shifts from sp²- to the sp³-hybridization and straightens. Iodinated lipids are more like saturated lipids than unsaturated ones, and can be viewed as being 'saturated by iodine' and not hydrogen—or partially-saturated by both.
The in vitro inhibition of breast cancer cells by iodine (I₂)—yet not iodide (I⁻)—has been explained by the reduction of prostaglandin E₂ synthesis consequent of membrane arachidonic acid iodination. I'd bet that I could find that study again if you'd like to read it.
'Prostaglandins are produced from AA [arachidonic acid] by the enzyme cyclooxygenase, indicating the presence of high levels of AA in breast tumors. It is possible that these high levels of AA, and the iodolipids formed from them, may explain the specific effect of I₂ in tumoral cells. This hypothesis is being explored in our laboratory.' ―Arroyo-Helguera
I didn't find much about iodinated PUFAs (apart from AA with 6-iodolactone), but considering that iodine is a halogen then Chlorine, Fluorine, Bromine, etc. would have similar binding capacities, and it really is much easier to find content about them.
So back to the topic, does a PUFA that has its double bounds iodinated/halogenated really start to behave more like a saturated fatty acid? Apparently so! If we use Linoleic Acid, with its 2 double bounds and melting point at -5°C, and saturate the double bounds with bromine, Linoleic Acid is now called tetrabromostearic acid, with a melting point of 115°C, and if we do this with Oleic Acid and its single double bound, we would get dibromostearic acid(9-10 dibromostearic acid).
The name is “converted” according to the number of carbons and the halogen present, so 18C UFAs would be known as somethingstearic, while if it were 16C it would be somethingpalmitic (such as dibromopalmitic). If we used iodine, as in the bromine examples, linoleic would become tetraiodostearic and oleic would become diiodostearic.
The theory is interesting, but what about in practice? Luckily for me, there's a study in which they compared a fat-free diet and a fat-free diet with the addition of tetrabromostearic acid (brominated linoleic acid) in terms of their ability to generate the classic symptoms of essential fatty acid deficiency(scaly paws, scaly or necrotic tails, and decreased growth). What were the results?
- Skin symptoms appeared somewhat earlier but were not more severe in the adults receiving the 3 per cent tetrabromostearic acid than in the animals receiving only the basal(fat-free) diet
- At the time of sacrifice, all rats receiving the basal diet(fat-free) or the diet containing 3 per cent tetrabromostearic acid exhibited the signs of essential fatty acid deficiency: scaly paws, scaly or necrotic tails, and decreased growth.
- Although the animals fed the tetrabromostearic acid (Table I) showed symptoms of fat deficiency, these animals had only a slight increase in cytochrome oxidase activity. This increase becomes evident when expressed on the basis of liver nitrogen, but the low number of observations does not allow the assignment of any significance to this oxidase activity. With this exception, however, liver enzyme activity in the animals fed the tetrabromostearic acid was almost exactly the same as that in animals fed the basal(fat-free) diet
- The addition of 9, 10,12,13-tetrabromostearic acid to the basal diet appears to have little effect on the development of fat deficiency, except possibly to reduce cytochrome oxidase activity.
This is just one study that I found interesting to mention because it involved a deficiency of essential fatty acids, but apparently confirms the idea that halogenated fatty acids behave more like saturated fats.
Cunningham, H. M., & Lawrence, G. A. (1977). Absorption and metabolism of chlorinated fatty acids and triglycerides in rats. Food and Cosmetics Toxicology, 15(2), 101–103
H O KUNKEL, J N WILLIAMS Jr. The effects of fat deficiency upon enzyme activity in the rat