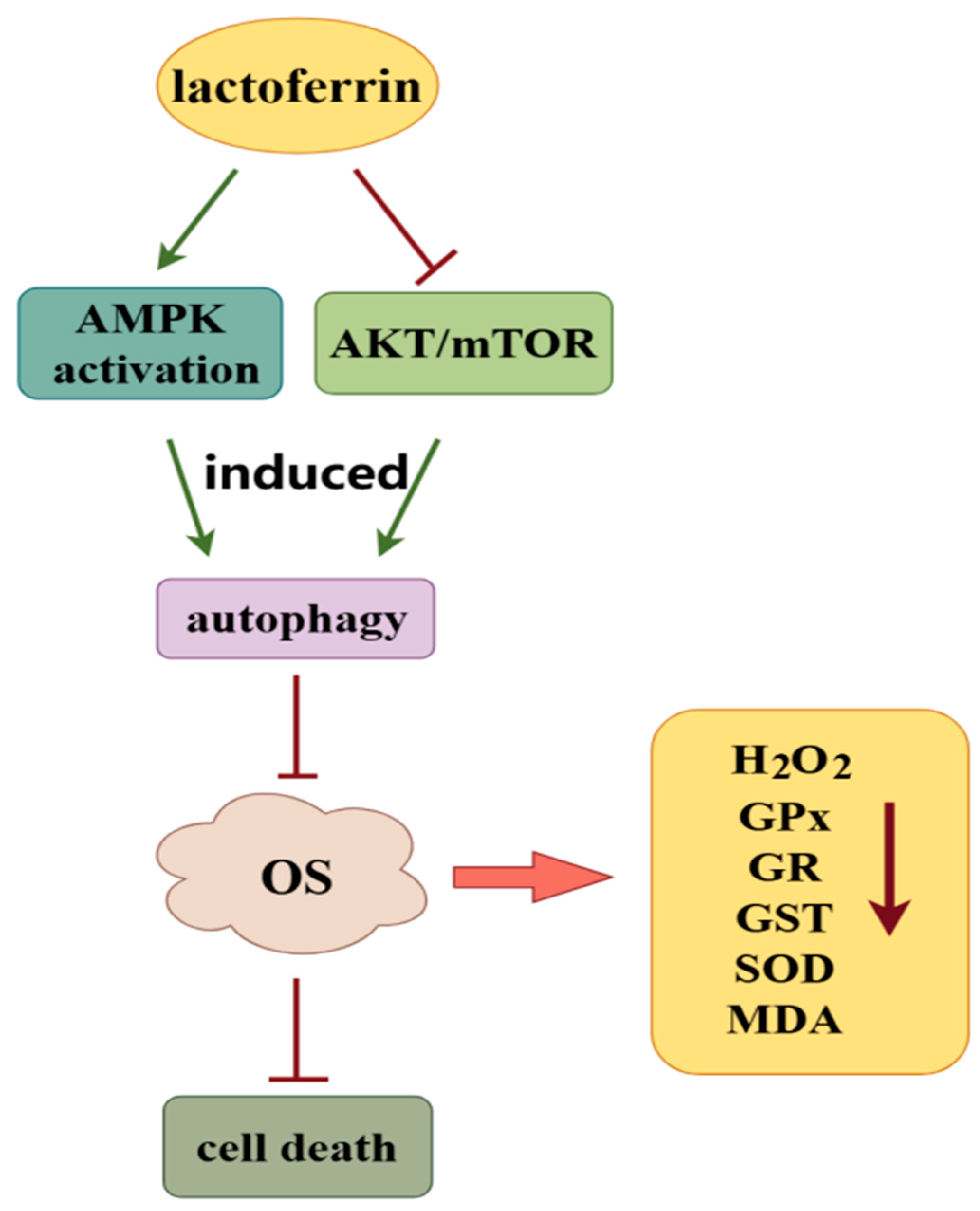
This post covers additional details about the function of Complex III and how it relates to other respiratory complexes, starting with nomenclature:
Complex III is also referred to as Cytochrome b-c1 complex, Ubiquinol–cytochrome c Oxidoreductase, or Cytochrome c Reductase.
Cytochrome b-c1 complex highlights the electron-transfer cytochromes representing each chain:
High-potential chain (↑)
Cytochrome c1 (+230 mV)
Iron-sulfur Protein (ISP) (+250 mV)
Low-potential chain (↓)
Cytochromes b
Cytochrome bL (low-potential heme: −30 mV)
Cytochrome bH (high-potential heme: +100 mV)
⠀
[image: 1757372015647-f56dbb6e-df24-45dc-973f-9e794ceae17b-image.png]
⠀(10.1016/j.bbabio.2012.11.008)
[It's a bacterial Complex III, but the core components in evidence remain the same. FeS clusters (ISC) are part of ISPs.]
Ubiquinol–cytochrome c Oxidoreductase highlights the overall reaction, where ubiquinol is the electron donor and cytochrome c the acceptor, and indicates the role of Complex III as oxidant to one and reductant to the other.
Cytochrome c Reductase emphasizes only its function as a cytochrome c reductant. Electrons can reach cytochrome c from sources other than Complex III, making this term less specific.
Then, Complex III reduces and Complex IV oxidizes cytochrome c :
Cytochrome c Reductase (Complex III)
↻ Cytochrome c
Cytochrome c Oxidase (Complex IV)
Calling Complex IV 'cytochrome c' for short is comparable to calling succinate dehydrogenase (SDH) 'succinate'.
Complex III is often used interchangeably with III₂. Both notations refer to the same enzyme, which exists as a dimer needed for proper function. A figure from an earlier post highlights one monomer in pink and the other in yellow:
[image: 1757372077953-528b59bc-fff9-4059-b87b-d60e0defc96b-image.png]
⠀(10.1038/s42255-023-00956-y)
These monomers are sometimes called protomers, implying that they are part of a larger complex and don't occur in isolation.
A Complex III dimer has 4 Q-sites in total (red circles), 2 per monomer, but located on different levels:
Qp (positive) [sometimes called Qo (outside)]
Qn (negative) [sometimes called Qi (inside)]
[image: 1757372105275-dfd0c642-edd3-4bec-8f7b-cf0c14ab50eb-image.png]
⠀(10.1016/j.bbabio.2012.11.008)
Electrons from UQH₂ oxidized at Qp-sites bifurcate into the high- and low-potential chains:
UQH₂ (2e⁻)
↳ 1e⁻ → [ISP → Cyt c1 →] Cyt c
↳ 1e⁻ → [Cyt bL → Cyt bH →] UQ/UQH•
So, one electron moves 'upward' (toward cyt c) and the other 'downward' (toward UQ/UQH•) from a Qp-site.
The short distance separating the two cytochromes bL allows electron transfer between monomers.
[image: 1757372015647-f56dbb6e-df24-45dc-973f-9e794ceae17b-image.png]
[image: 1757372215623-f1e9aed8-4263-4734-ad00-733b0713702b-image.png]
⠀(10.1016/j.bbabio.2008.04.022)
(Here they call the Qp-site 'Center P' and the Qn-site 'Center N'. Damn it.)
This bL–bL interaction lets the Complex III dimer reroute electrons when needed, preventing incomplete oxidation of UQH₂ at the Qp-site and consequently reducing the risk of ROS production.
Complex III releases superoxide to the matrix and the cytosol, but most of it originates from the susceptible Qp region:
[image: 1757372273140-adcfbb6f-e999-46d6-99fa-5e11e0823374-image.png]
⠀(10.1074/jbc.M407715200)
However, electron accumulation anywhere can affect the Qp regions indirectly, so increasing the number of possible electron acceptors through inter-monomer communication gives Complex III flexibility.
Monomer A
Monomer A
Monomer B
Monomer B
UQH₂
{Qp/Qo}
(Empty)
1e⁻
1e⁻ ↓
↓
ISP ◯
bL ◯
⇄
◯ bL
◯ ISP
↓
⇅
⇅
↓
c1 ◯
bH ◯
◯ bH
◯ c1
↓
⇅
⇅
↓
c ◯
UQ ◯◯
{Qn/Qi}
◯◯ UQ
◯ c
c ↵
UQH• ◯ ↵
or
↳ ◯ UQH•
High-potential chain
Low-potential chain
Low-potential chain
High-potential chain
To avoid mixing up the order of cytochromes b, this may help:
Qo - bL - bH - Qi
Which reads:
oL-Hi
An inverted Low next to a High. Let the trauma sink in.
I find the obsession with reverse electron flow at Complex I out of proportion. It does happen, but is energetically unfavorable, needing an excess of electrons in the UQ pool along with elevated membrane electrical and chemical potentials to force Complex I to run in reverse. In contrast, it doesn't seem to bother the obsessed how the same excess electrons in the UQ pool might affect other UQ-dependent respiratory complexes that operate nearer the UQH₂/UQ potential, making them less resistant to backflow than Complex I.
Like Complex I, Complex III relies on UQ (the oxidized form) to function. As redundant as it may seem, Complex III uses UQ to oxidize UQH₂, but only partially because the other half of electrons go to cytochrome c. Whether UQH₂ is produced at Complex I or III, its protons (H⁺) are derived from the matrix of mitochondria.
Qp-sites are traditionally viewed as UQH₂-oxidizing centers (where electrons enter Complex III), while Qn-sites are viewed as UQ-reducing centers (where electrons leave Complex III). However, electrons can also enter via Qn-sites, especially when UQH₂ is in excess.
The redox state of cyt bH changes the behavior of its Qn-site:
Oxidized cyt bH is an acceptor and favors UQH₂ binding to gain an electron.
Cyt bH³⁺ ◯ ↶ UQH₂
Reduced cyt bH is a donor and favors UQ binding to lose an electron.
Cyt bH²⁺ ↷ ◯◯ UQ
This change in affinity according to the state of cyt bH reduces Complex III dependence on appropriate levels of UQH₂ and UQ. In either case an ubisemiquinone [◯ UQH•] is formed and stabilized at the Qn-site.
The occupancy of Qn-sites affect the activity of Qp-sites via ISP. Possible scenarios:
Qn-site A
Qn-site B
Outcome
Qp-sites A and B
UQH•
UQH•
→
1 active
Empty
Empty
→
1 active
UQH•
Empty
→
2 active
Empty
UQH•
→
2 active
Due to intense activity, the first scenario is more common, which limits oxidation capacity but prevents incomplete UQH₂ oxidation and premature electron leakage to oxygen around the problematic Qp-sites.
Since monomers interact, an electron entering via a Qn-site can transfer to the opposite monomer, promoting UQ binding there to accept it. This clears the original monomer for unimpeded electron flow from a Qp-site while stabilizing another terminal acceptor on the neighboring monomer. The result is technically known as 'bi-winning': win here, win there.
[image: 1757372854255-15b46985-2907-420a-99c7-03fa33ed151b-image.png]
⠀(10.1016/j.bbabio.2008.04.008)
The figure below shows UQH₂ again entering a Qp‑site for oxidation, but in contrast to before, the low-potential chain of this monomer wasn't primed for oxidation. The path to its Qn‑site is now blocked, but the electron can divert to the opposite monomer to complete the process.
[image: 1757372876452-37ed3c35-9d0a-4efd-aca3-33e68c65fa49-image.png]
⠀(10.1016/j.bbabio.2008.04.008)
These inter-monomer interactions often take place within larger structures, as it's common for a Complex III dimer to associate with other respiratory complexes. For example, most Complex I occurs with Complex III in a supercomplex:
[image: 1757372946934-0c20f30f-de55-49c8-b13b-79ab85e9f389-image.png]
⠀(10.1074/jbc.M106474200)
Composition
Frequency
I+III₂+IV
~54%
I+III₂
~17%
I+III₂+IV₂
~9%
I+III₂+IV₂+IV
~3%
I+III₂+IV₂+IV₂
~1%
I (free)
~16%
This is how Complex III₂ associates with Complex I, in the presence or absence of Complex IV:
[image: 1757372995228-e937442c-42b7-4427-8e2d-fc27b6d01249-image.png]
⠀(10.1038/nature19774)
I+III₂+IV (a,b)
I+III₂ (c)
As you can tell, the 'heel' of Complex I isn't fully turned against Complex III (as often represented in figures), nor is the heel directly facing Complex III. Instead, Complex I and Complex III are arranged in parallel, and one of the Complex III monomers ends up adjacent to Complex I, gaining privileged access to the Q-tunnel of Complex I:
[image: 1757373050908-640d7f1c-dbeb-464c-9ad9-df72b0407156-image.png]
⠀(10.1016/j.molcel.2019.07.022)
From the legend, this is a cross-section of the I+III₂ supercomplex through the membrane domains and viewed from the matrix.
Proximal and distal refer to two lipid-filled cavities on opposite sides of the Complex III dimer: each cavity gives access to the Qp-site of one monomer and Qn-site of the other, shown by the alternate 'dashed wedges' on a lower plane and the 'solid wedges' on a higher plane (which includes the Q-tunnel). The proximity is relative to the Q-tunnel (a source of UQH₂ for Complex III to oxidize).
It helps to picture the Q‑tunnel at the heel of Complex I, but the tunnel and its opening are oriented more to the side than to the back:
[image: 1757373152795-84e43691-17e6-4e4d-b11f-c8c7ed628448-image.png]
⠀(10.1080/09168451.2020.1747974)
When Complex IV joins the supercomplex, it forms an autonomous respiratory unit capable of completing respiration, hence the term 'respirasome'.
[image: 1757373189597-9fa6d06c-e80f-4f1a-be10-d6c1b57c571d-image.png]
⠀(10.1016/j.sbi.2020.01.004)
[image: 1757373216932-1ac3fa39-599c-458a-8893-418bf8b6f94a-image.png]
⠀(10.1038/nature19774)
Rotating it to coincide with the view of the previous figure:
[image: 1757373253787-8c23a569-d87e-4298-94b0-b89052a70d52-image.png]
IV: pink
IIIb: dark green
IIIa: light green
I: blue
The authors speculate that occlusion of the distal cavity by Complex IV may be advantageous: because the Q-cycle involves formation of ubisemiquinone radicals, partial closure of the cavity while Complex IV consumes local oxygen may protect unstable intermediates from adverse reactions. However, they also acknowledge that the occluded Qn-site is not a major ROS producer and that the Qp-site in the same cavity may be inactive.
For mobile carriers that connect these respiratory complexes, some argue for the existence of dedicated UQ pools for NAD-linked and FAD-linked respiration.
[image: 1757373298373-c97d77bb-e630-4c8c-9dba-3224505d1b89-image.png]
⠀(10.1016/j.freeradbiomed.2021.03.010)
Relying only on UQ trapped within I+III₂(+IV) would limit reoxidation of Complex I–derived UQH₂ to the proximal cavity of Complex III₂, because of its exclusive access to the Q-tunnel. Nonetheless, these respiratory complexes have their quinone sites exposed to the supercomplex exterior and can exchange with the free UQ pool, so they're not restricted to local UQ and can respond to mitochondrial conditions. This flexibility is particularly important given that Complex I can outpace Complex III. More information here.
Because the Qp-site of monomer A shares a cavity with the Qn-site of monomer B, reoxidized UQ can return to Complex I to bring in more electrons, exchange with the free UQ pool around the supercomplex (and indirectly affect the distal cavity), or perhaps be reused in the same cavity as an electron acceptor. This last possibility isn't futile, since part of electrons are removed via cytochrome c.
Monomer A
Monomer A
Monomer B
UQH₂
↷
UQ
1e⁻
1e⁻ ↓
⇅
ISP
bL
bH
↓
⇅
⇅
c1
bH
bL
↓
⇅
c
UQH•
High-potential chain
Low-potential chain
Low-potential chain
If UQ turnover is insufficient, an elevated UQH₂/UQ ratio can favor ROS production and reversibly deactivate Complex I or promote its degradation for supercomplex remodeling, freeing some of the III₂+IV to serve FAD-dependent complexes. These can be adaptive. Recall that substrates more reliant on FAD-linked respiration (↓ATP/O) demand additional UQ turnover.
I ↮ III₂+IV
SDH/ETFDH/etc. + III₂+IV
[image: 1757373484287-023b11f6-9806-4f94-90f2-049cb214d084-image.png]
⠀(10.1016/j.bbabio.2023.148977)
It's complicated at times, but when a problem appears to run counter to the oversimplified and sensationalist take of bioenergetic gurus, it's probably a good sign.
⠀
Herrero Martín, J. C., Salegi Ansa, B., Álvarez-Rivera, G., Domínguez-Zorita, S., Rodríguez-Pombo, P., Pérez, B., ... & Formentini, L. (2024). An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis. Nature metabolism, 6(2), 209-225. https://doi.org/10.1038/s42255-023-00956-y
Muller, F. L., Liu, Y., & Van Remmen, H. (2004). Complex III releases superoxide to both sides of the inner mitochondrial membrane. Journal of Biological Chemistry, 279(47), 49064-49073. https://doi.org/10.1074/jbc.M407715200
Murai, M. (2020). Exploring the binding pocket of quinone/inhibitors in mitochondrial respiratory complex I by chemical biology approaches. Bioscience, biotechnology, and biochemistry, 84(7), 1322-1331. https://doi.org/10.1080/09168451.2020.1747974
Hernansanz-Agustín, P., & Enríquez, J. A. (2021). Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radical Biology and Medicine, 167, 232-242. https://doi.org/10.1016/j.freeradbiomed.2021.03.010
Covian, R., & Trumpower, B. L. (2008). Regulatory interactions in the dimeric cytochrome bc1 complex: the advantages of being a twin. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1777(9), 1079-1091. https://doi.org/10.1016/j.bbabio.2008.04.022
Parey, K., Wirth, C., Vonck, J., & Zickermann, V. (2020). Respiratory complex I—structure, mechanism and evolution. Current opinion in structural biology, 63, 1-9. https://doi.org/10.1016/j.sbi.2020.01.004
Xia, D., Esser, L., Tang, W. K., Zhou, F., Zhou, Y., Yu, L., & Yu, C. A. (2013). Structural analysis of cytochrome bc1 complexes: implications to the mechanism of function. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1827(11-12), 1278-1294. https://doi.org/10.1016/j.bbabio.2012.11.008
Letts, J. A., Fiedorczuk, K., Degliesposti, G., Skehel, M., & Sazanov, L. A. (2019). Structures of respiratory supercomplex I+ III2 reveal functional and conformational crosstalk. Molecular cell, 75(6), 1131-1146. https://doi.org/10.1016/j.molcel.2019.07.022
Letts, J. A., Fiedorczuk, K., & Sazanov, L. A. (2016). The architecture of respiratory supercomplexes. Nature, 537(7622), 644-648. https://doi.org/10.1038/nature19774
Guaras, A., Perales-Clemente, E., Calvo, E., Acín-Pérez, R., Loureiro-Lopez, M., Pujol, C., ... & Enríquez, J. A. (2016). The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell reports, 15(1), 197-209. https://doi.org/10.1016/j.celrep.2016.03.009
Covian, R., & Trumpower, B. L. (2008). The dimeric structure of the cytochrome bc1 complex prevents center P inhibition by reverse reactions at center N. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1777(7-8), 1044-1052. https://doi.org/10.1016/j.bbabio.2008.04.008
Schägger, H., & Pfeiffer, K. (2001). The ratio of oxidative phosphorylation complexes I–V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. Journal of Biological Chemistry, 276(41), 37861-37867. https://doi.org/10.1074/jbc.M106474200
Nesci, S., Algieri, C., Trombetti, F., Fabbri, M., & Lenaz, G. (2023). Two separate pathways underlie NADH and succinate oxidation in swine heart mitochondria: Kinetic evidence on the mobile electron carriers. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1864(3), 148977. https://doi.org/10.1016/j.bbabio.2023.148977